Oxidizing and non-oxidizing biocides [Download this white paper as PDF file]
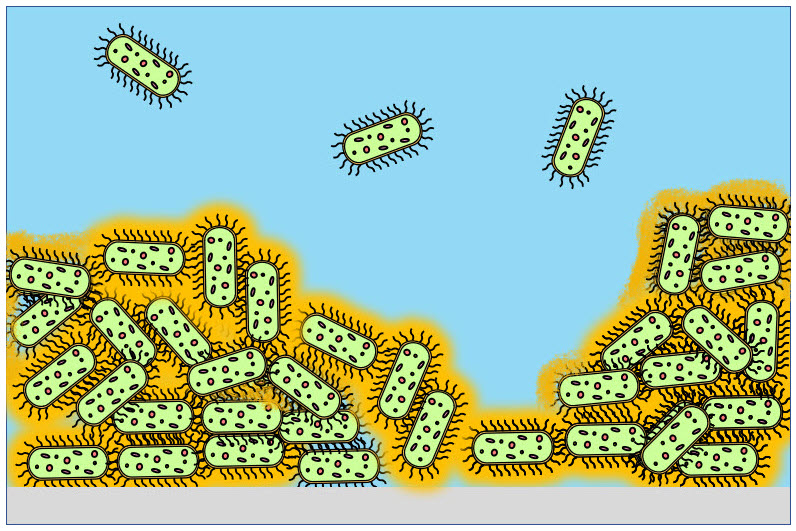
Adopting practices to keep bacterial growth under control is required in all industrial plants that use water or other liquids, since in this kind of environment microorganisms can proliferate rapidly, causing several problems. When the conditions (such as presence of nutrients, low flow rate and suitable temperature) allow it, bacterial growth on internal surfaces of pipes and tanks occurs easily. In this case, the development of the so-called "biofilm" takes place. This is a phenomenon of primary importance, since 90% of the total number of bacteria lives in biofilm, while just 10% is free in the liquid (see Fig. on the right). Furthermore, bacteria that live in biofilm produce exopolymeric substances (EPS) that form a protective layer, increasing up to 1000 times their resistance to external agents.
In order to eliminate communities of microorganisms developed inside the plants, physical treatments (UV, ultrasound..) or chemical treatments (biocides and sanitizers) can be applied. An ideal sanitation treatment should be:
- dispersant: destroy the protective layer of EPS, allowing an easier penetration of the biocide into biofilm;
- microbiocide: reduce the microbiological load eliminating bacteria, thanks to the chemical action of biocides;
- bacteriostatic: keep as low as possible the concentration of microorganisms.
Dispersants
Dispersants, or biopenetrants, are compounds that selectively react with the deposit on the surfaces of pipes and with the biofilm matrix, composed of exopolymeric substances. Biopenetrants increase the efficacy of biocide treatment, because they destroy the polymeric matrix and favor biocides penetration within biofilm. Destroying the protective shelter of biofilm, bacterial cells are directly exposed in solution to biocides attack, therefore cells are easily killed.
Moreover, thanks to dispersants, biocides are not consumed by the reaction with EPS, so they can work completely against bacteria; this allows to reduce biocides dosage, and thus its cost.
The chemical structure of dispersants can be very different: anionic or cationic compounds, non-ionic polymers or enzymes. The use of enzymes in water treatment is growing, thanks to the increased awareness about the environmental impact of biocides treatment. Indeed, enzymes are fully biodegradable proteins, and does not lead to the production of toxic by-products. The main disadvantages of enzymes are the cost, that is very high due to the biotechnological process required to produce them, and the susceptibility to particular conditions (like extreme pH, high temperature, highly oxidizing environment) that can denature them.
However, it is important to consider that biopenetrants can support but not replace biocides, for what concerns bactericidal action. Usually they are dosed in the system before biocides, in order to expose bacteria and eliminate them more efficiently. If not used properly, dispersants release in solution a huge number of bacteria from biofilm, resulting in a counterproductive effect.
Biocides
Biocides are chemical compounds used in water treatment to eliminate or reduce bacterial load; they are potentially dangerous compounds, so stringent laws regulate the dosage and maximum concentration allowed in wastewater. The correct use of biocides in European Union is described in the BPR (Biocidal Products Regulation - UE regulation 528/2012) and the authorization for the use of biocides in each specific application is granted by the competent authority, the ECHA (European Chemicals Agency). In the USA, this activity is carried out by the EPA (Environmental Protection Agency), that wrote the FIFRA (Federal Insecticide Fungicide and Rodenticide Act), a document that includes all the regulations concerning biocides, here defined as "antimicrobial pesticides". In general, in every country there are laws or regulations about biocides; these are constantly updated and implemented in order to limit as much as possible the use of compounds potentially harmful to the environment.
An ideal biocide has several properties:
- good efficacy even at low concentration;
- fast action;
- applicability over a wide range of operating conditions (pH, temperature, etc);
- broad range of activity (efficacy against an elevated number of bacterial species);
- low environmental impact (absence of organic solvents and heavy metals, reduced formation of toxic by-products, low persistence in water);
- safety for operators;
- low cost.
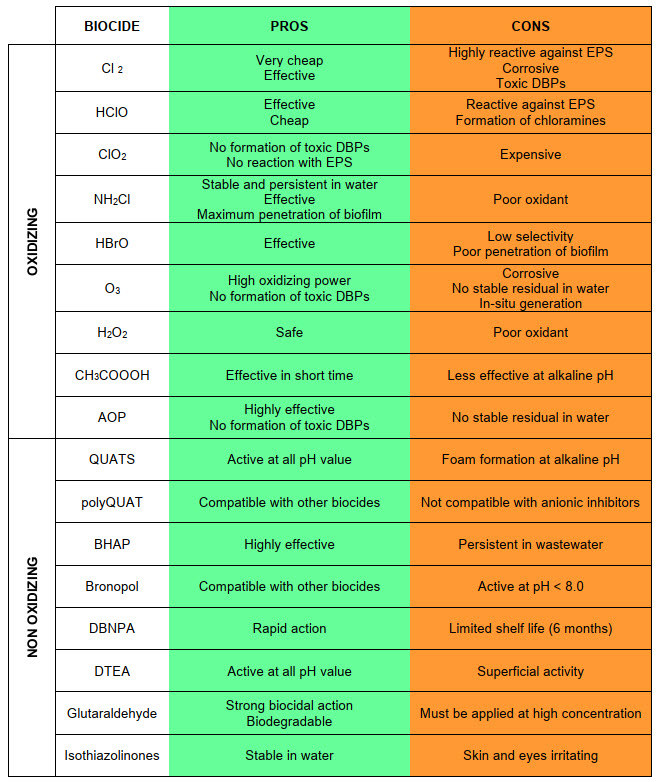
As later described in detail (Tab. at the end), no compound will ever satisfy all requirements, as well as no compound will work equally well in all applications - the choice of the treatment, indeed, shall be always evaluated on the specific application. To this aim, the following factors should be considered:
- frequency and contact time of the treatment;
- use of additives or biopenetrants;
- flow rate in the system;
- microbiological load;
- compatibility with the system;
- interference with other compounds, like inhibitors or contaminants.
Even considering all the points mentioned above, it shall be notes that it is impossible to predict exactly the efficacy of the applied treatment - that is the reason why biofilm monitoring is the best approach to verify the real result of a disinfection treatment.
|
The Sensors developed by ALVIM enable to monitor biofilm growth and removal in real time, and therefore to evaluate if the disinfection protocol is applied correctly. |
Generally speaking, biocides are highly reactive compounds, that rapidly degrade in by-products called DBP (Disinfection By- Products). They are available on the market in a huge number of formulations, divided into two macro-categories, oxidizing and non-oxidizing biocides.
Oxidizing biocides
Oxidizing biocides (OBs) are the most widely used for water treatment, due to the number of advantages they offer - they are cheap, with fast action and they are effective even at relatively low concentration.
OBs are highly reactive and, as a consequence, poorly selective. Indeed, OBs are active against a broad range of microorganisms, with several modes of action. For this reason, oxidizing biocides do not cause resistance. The main deactivation mechanism of microbial activity is an electron transfer reaction - the biocide is reduced, and the organic compounds like proteins, lipids or fatty acids, essential constituents of the cell, are oxidized. Based on the same mechanism, the biocide can react with the exopolymeric substances that cover biofilm and protect bacteria.
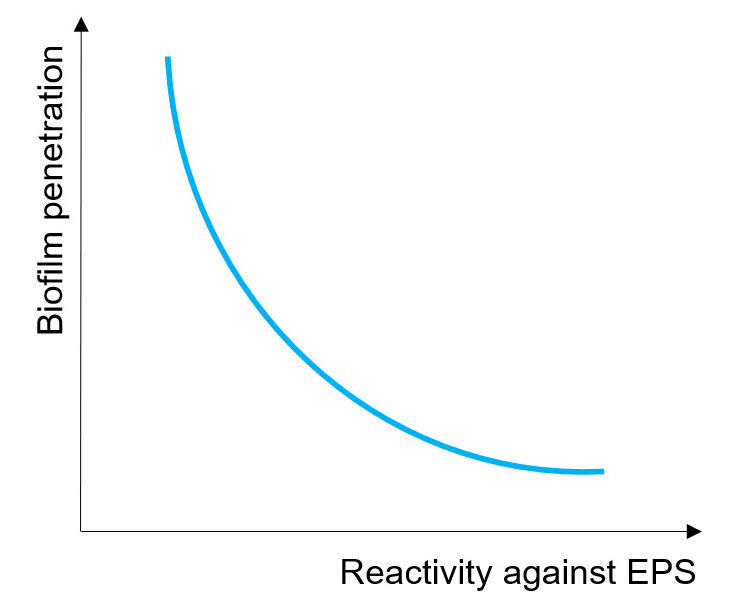
Some oxidizing biocides preferentially react with EPS layer, so they get consumed before they penetrate deeply inside biofilm. Others are less reactive against EPS, therefore they penetrate easily biofilm, eliminating bacteria more efficiently. It can be stated that the reactivity against exopolymeric substances is inversely proportional to the capability to penetrate biofilm (see Fig. on the right)
In general, OBs are very reactive and can interact also with additives, contaminants or other organic compounds present in the system. Since they are affected by the total oxidizing demand of the system, usually they are dosed in excess, to ensure an active residue in the water over time.
The main disadvantage of oxidizing biocides is the tendency to induce corrosion on metals, because of their redox potential. For this reason, they are often used in combination with corrosion inhibitors, chemical compounds that reduce the electrochemical degradation of metals by anodic or cathodic inhibition. Moreover, OBs are affected by the variation of some parameters like pH and temperature, so they are sensitive to the operative conditions of the system.
The oxidizing biocides more commonly used are chlorine- and bromine-based compounds (Cl2, hypochlorous acid, sodium hypochlorite, chlorine dioxide, hypobromous acid, active bromide, etc.), ozone, hydrogen peroxide and peracetic acid.
|
A treatment with oxidizing biocides increases the ORP value of the system, which is a parameter, detected by ALVIM Sensors - so they allow to verify the correct dosage and distribution of the biocide in a plant. |
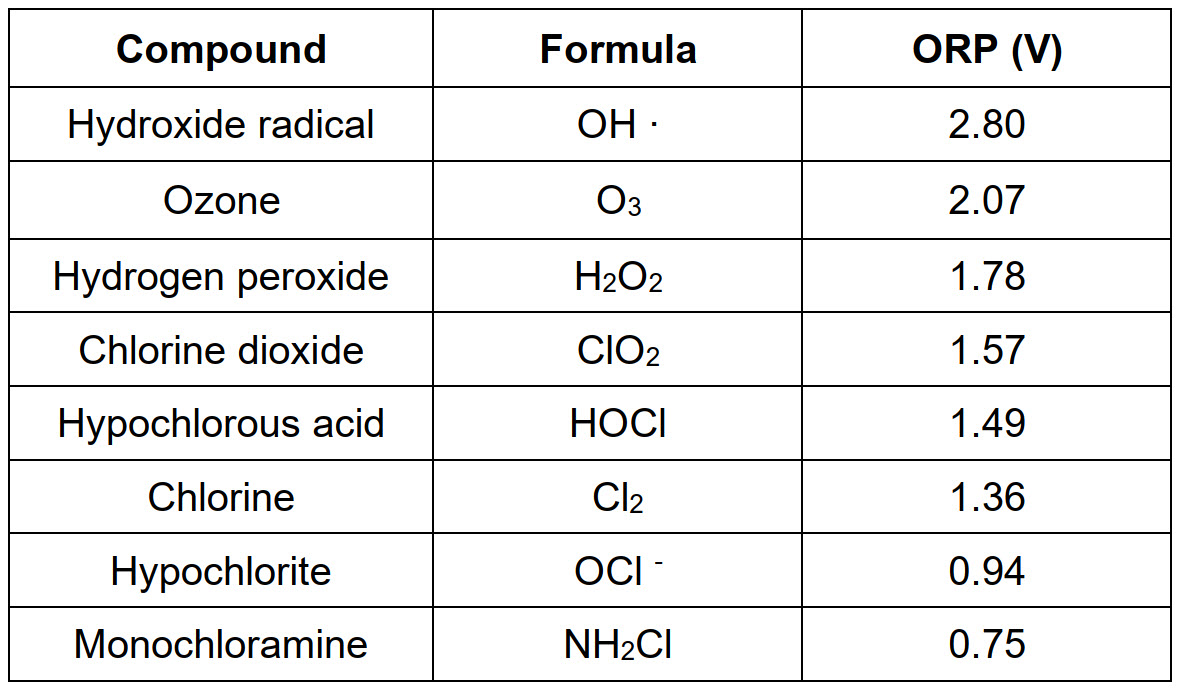
In the following table some OBs, in decreasing order of oxidizing power, are reported. Indeed, the redox potential (ORP) measures the tendency to gain electrons. A high ORP value means that the compound is easily reduced, i.e. it has a strong oxidizing power.
Chlorine
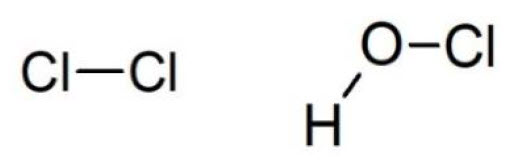
Chlorine is the most ancient method used for chemical disinfection of water. It is still used because it is very cheap, and it has a good effectiveness. The biocidal action of chlorine is not directly carried out by gaseous molecule of Cl2, but rather by compounds formed when the gas hydrolyzes. Indeed, in water chlorine undergoes the hydrolysis reaction (see below), that forms hypochlorous and hydrochloric acid:
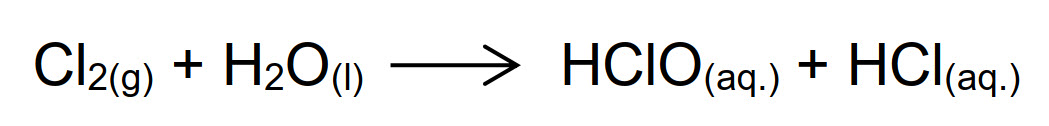
Therefore, the redox reactions with biocidal effect occur between hypochlorous acid (in equilibrium between HClO and ClO- forms) and components of the bacterial cells. Hydrochloric acid instead is responsible for the decrease in pH of the system - the higher the pH, the more chlorine will be needed to eliminate bacteria.
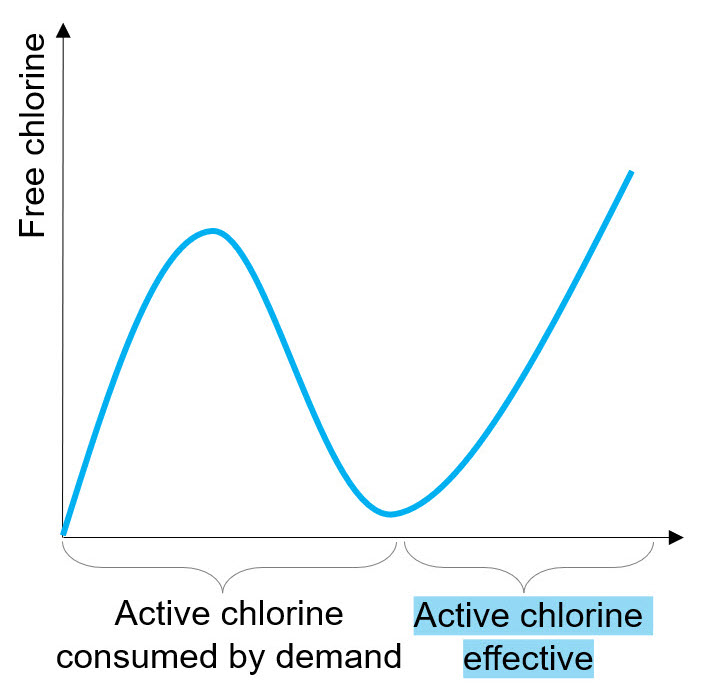
Chlorine vigorously reacts with contaminants, organic compounds or other additives that could be present in the system, as well as with exopolymeric substances that cover biofilm. Chlorine is characterized by kinetics of reaction with other molecules much higher than kinetics of diffusion inside biofilm, so it tends to react preferentially with abiotic components of EPS. To ensure a deep penetration of the treatment inside biofilm, a medium to high dosage is therefore required. Indeed, chlorination is effective only if biocide concentration is higher than the demand of active chlorine (see Fig. on the right).
The acidity of the system, due to the formation of hydrochloric acid, is associated with an elevated corrosion rate - and this is definitely a disadvantage of chlorine. In addition, chlorine forms by-products such as adsorbable organic halogens (AOX), trihalomethane (THM) and haloacetic acid (HAA) that are toxic in water and environmentally harmful. The acceptable concentration of residual chlorine is highly regulated because of its toxicity, and for the same reason there are strict safety protocols for workers that handle and stock gaseous Cl2. The maximum concentration of residual chlorine in water is 0.2 mg/L in EU, 4 mg/L in the USA.
For these reasons, despite efficacy and low cost, Cl2 nowadays is not largely used in water sanification treatment. On the contrary, hypochlorous acid (HClO) is widely applied as oxidizing biocide - in water it is in equilibrium with hypochlorite anion (ClO-), its conjugated base. The ratio between the concentration of those species is determined by the acid dissociation constant pKa = 7.5 for the following reaction:
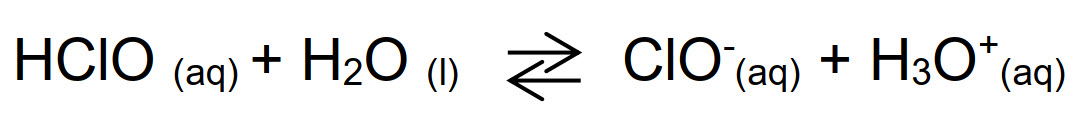
This equilibrium is pH-dependent, so system conditions will affect the ratio HClO/ ClO-, defined as "free chlorine".
Hydrochloric acid is more effective than hypochlorite - since it is smaller and neutral, it can penetrate cell membrane better. In the pH range 6.5-7.5 the dominant chemical species is ClO- anion, up to 100 time less effective than its conjugated acid, so a biocidal treatment applied at this pH is not optimized.
Hypochlorite can be added as a liquid (concentrated solution of NaClO), as a solid (tablet of Ca(ClO)2) or it can be generated in-situ; there are advanced systems for OSHG (On Site Hypochlorite Generation) based on NaCl electrolysis:

The gaseous chlorine generated in water is then transformed in hypochlorous acid, as previously described. The disadvantage of this method is the formation of hydrogen as by-product, which is potentially dangerous because it is explosive.
Chlorine dioxide
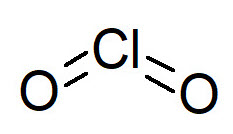
Unlike molecular chlorine, that hydrolyzes in water, ClO2 is a gaseous compound very soluble in water:
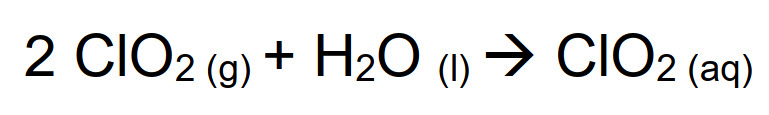
However, ClO2 (aq) aqueous solution is not very stable and disproportion reaction occurs with time, forming hypochlorous and chloric acid:

Chlorine dioxide is less reactive than Cl2, because it selectively reacts with reduced sulfur compounds, with secondary and tertiary amines and with some highly reduced organic compounds. ClO2 doesn't react with phenols, resulting from the decomposition of humic acids, and therefore it does not form trihalomethane nor other DBPs, molecules that usually have a high environmental impact.
ClO2 does not react with EPS either, so it is not consumed and it can penetrate and eliminate biofilm more efficiently. As a consequence, chlorine dioxide has an elevated bactericidal efficiency on broad spectrum, constant in the pH range 4-10.
Monochloramine
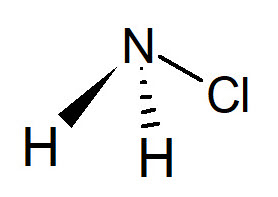
NH2Cl has been used for potable water treatment for 100 years, and today is increasingly used for hot water disinfection in hospitals, large facilities and industrial plants. In North America, this chemical is commonly used also for treating water distribution networks. The World Health Organization (WHO) indicates that monochloramine has a great disinfection efficacy and it is one of the best chemicals to prevent the risk of Legionellosis. Compared to chlorine, NH2Cl is more stable and persistent in water, ensuring a more effective diffusion even in stagnant areas. According to WHO, residual monochloramine is authorized in potable water up to 3 mg/L, while the maximum concentration accepted by US EPA is 4 mg/L - much more than the concentration allowed for residual chlorine.
The reaction product of condensation between hypochlorous acid and ammonia is a chlorinated amine, mono- bi- or tri-substituted depending on pH and on stoichiometric ratio of precursors. In order to ensure a quantitative formation of monochloramine, reaction must occur at pH > 7.2, while at lower pH also the secondary and tertiary amines form, as described in Fig. below:
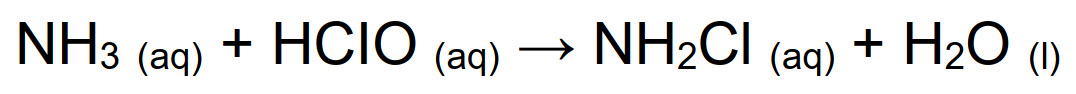
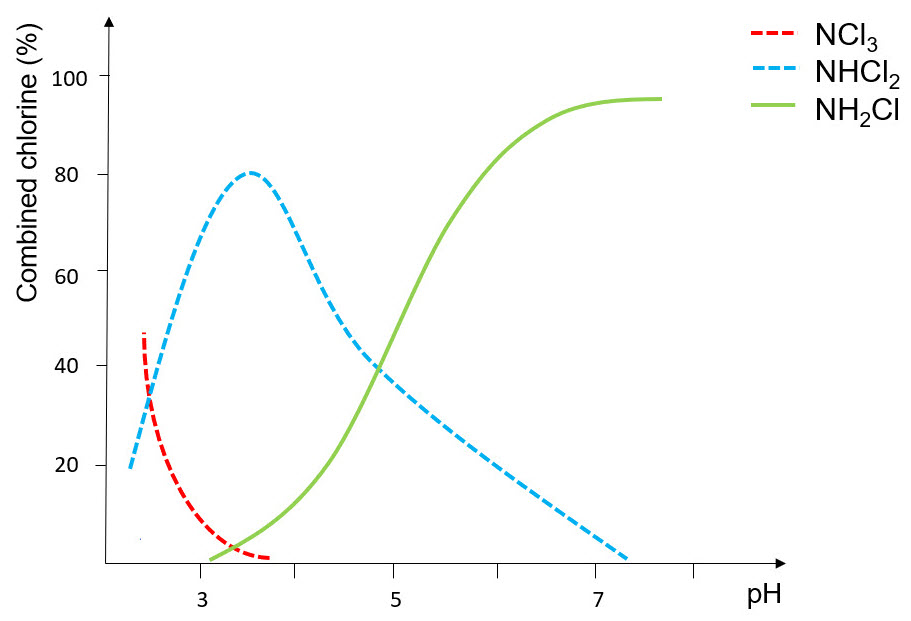
Compared to other chlorine-based chemicals, monochloramine has a lower oxidizing strength (see previous Tab.) and it is an highly stable molecule. This allows the maximum penetration inside biofilm, reaching bacteria and pathogens as Legionella. Because of the minor reactivity and the high stability in water, NH2Cl is not completely consumed in the first organic layer, but it can reach the deepest layer of biofilm, where it still has bactericidal action. As a mild oxidizing agent, monochloramine is compatible with all meta llic and plastic materials in the piping system, that are not corroded.
|
The reason for the minor reactivity and major stability of NH2Cl, compared to other chlorine-based biocides (HOCl, ClO2), is the different molecular structure -monochloramine contains a strong covalent N-Cl bond, hard to break. The O-Cl bond is different because oxygen is very electronegative, so it attracts the electronic density and it polarizes the bond. This makes chlorine more prone to react with other molecules as an oxidant. The mechanism of biocidal action is the specific reaction between NH2Cl and -SH groups of cysteines, present in the proteins of bacterial cells or in enzymes involved in cellular pathways. |
Bromine
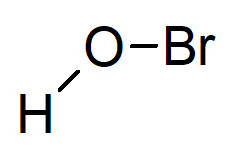
Molecular bromine Br2 is an extremely toxic and reactive gas with high biocidal efficiency, but it is not applied in disinfection treatments because it is too hazardous. Other derivatives of bromine are used instead, such as hypobromous acid (HBrO) that undergoes to the following dissociation equilibrium:
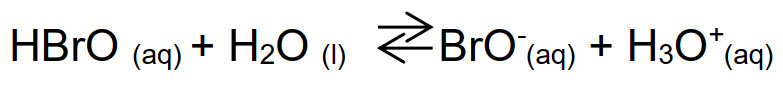
The equilibrium hypobromite/hypobromous acid has an acid dissociation constant pKa= 8.7, higher than that of chlorine. It means that HBrO has a minor tendency to dissociate than HClO. Indeed, at the same pH the concentration of bromine conjugated base (BrO-) will be lower than ClO-, as it can be seen in Fig. below. A bromine-based biocide will be more effective than the analogous chlorine-based, because the acid has the stronger biocidal action.
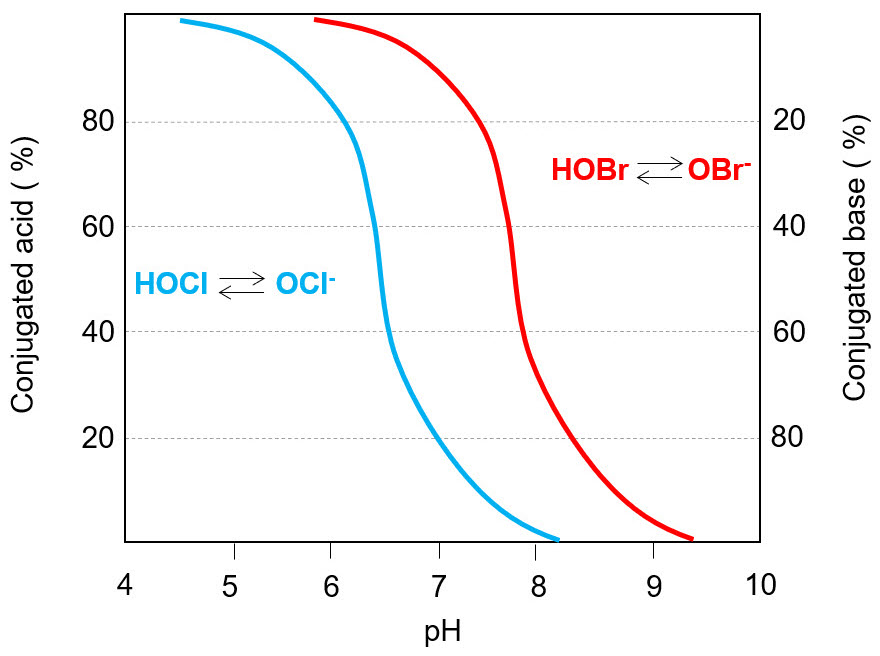
The reactivity of bromine-based compounds is high, so the selectivity against other compounds or contaminants in solution ns is poor. Similarly to chlorine, bromine-based biocides react effectively with exopolymeric substances (EPS), and this determines a low capacity to penetrate biofilm.
Bromine-based chemicals with bactericidal action are usually generated in-situ, from stabilized precursors as sodium bromide (NaBr), through electrolysis or adding an activating agent as O3 or NaClO.
Ozone

Ozone (O3) is a gas with pungent odor, used for the first time as disinfectant in an industrial plant in 1906. It has a high redox potential (ORP = 2.07 V), quantifying its strong oxidizing capacity.
For this reason, ozone rapidly reacts also with metals, accelerating their corrosion. O3 degrades faster in water than in air, reducing to molecular oxygen O2 at the end of disinfection process. It is considered a safe biocide because it does not generate toxic compounds with strong environmental footprint (THM, HAA) and, unlike other oxidizing biocides, it does not leave any odor or flavor, so it is often applied in the food industry.
|
The bactericidal action of ozone arises from its reaction of cycloaddition (Criegee mechanism) with double bonds of fatty acids present in bacteria cell walls. In a protic solvent as water, the reaction will proceed breaking fatty acids into carboxylic residuals. The oxidation of the non-polar tail of fatty acids leads to change in membrane permeability and to release in solution of bacterial cell content, resulting in cell destruction (bacterial lysis). Ozone reacts also with aromatic compounds activated by electron-donor substituents through electrophilic addition, and with other organic compounds, partially oxidizing them. |
The stability of ozone is inversely proportional to its reactivity, and it depends on various parameters such as temperature, pH, carbonate concentration, type of microorganisms, etc. In standard conditions (T = 25℃, p = 1 atm, water) O3 has a lifetime of 25 minutes, decreasing as the temperature increases and increasing as carbonates increases, because CO32- behaves as inhibitor of radical chain reaction.
Because of the reactivity and low solubility of ozone in liquid, it is almost impossible to maintain a stable and persistent residue in water. To get a complete biocide treatment, repeated injections are required - however, even a low concentration is enough, thanks to the strong oxidizing power. Ozone is a highly reactive oxidant, so it is unstable and it cannot be stored as a pure gas. For this reason, O3 must be generated in situ, through ozonation processes. The corona discharge method is the most widely used - a high frequency electric field, with enough energy to break the covalent bond O-O of molecular oxygen, is applied to air. Radical oxygen atoms obtained from splitting recombine with another oxygen molecule to form O3, as described below:
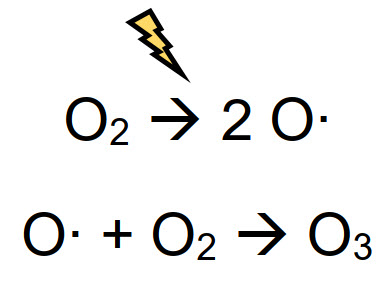
The concentration of ozone produced through corona discharge generators is 0.5-3.0% in weight, while it is up to 5.0% if a pure oxygen source in used, instead of air. It is clear that a sanitation treatment with ozone is more expensive than the use of other biocides like hypochlorite.
Hydrogen peroxide
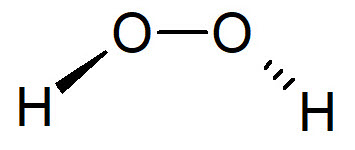
Hydrogen peroxide (H2O2) is an oxidizing biocide used almost always in combination with stronger chemicals, for the main purpose of potable water disinfection. H2O2 is also adopted in the food industry because it does not generate harmful DBPs when decomposes. The use of H2O2 in disinfection treatment is extensive, in 2019 the global market was about 4.8 billions dollar.
Peracetic acid
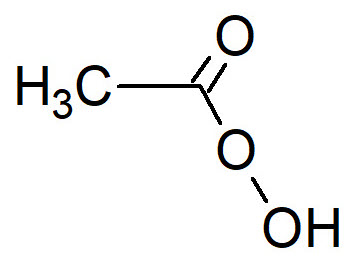
Peracetic acid (PAA) is an organic compound with formula CH3COOOH and it is an extremely powerful biocide, that acts in short time. It is a transparent liquid that can be directly added to the solution, or it can be obtained by acid catalyzed reaction between hydrogen peroxide and acetic acid.
Peracetic acid contains the peroxidic group -O-O- that generates free radicals through homolytic scission of bonds. Radicals are highly reactive but the biocidal effectiveness of PAA decreases as pH raises, so it is not suitable for use in alkaline systems.
Peracetic acid contains the peroxidic group -O-O- that generates free radicals through homolytic scission of bonds. Radicals are highly reactive but the biocidal effectiveness of PAA decreases as pH raises, so it is not suitable for use in alkaline systems.
AOP
An AOP (Advanced Oxidation Process) is a physical-chemical method that exploits the reactivity of oxidizing molecules to generate in-situ other highly biocidal species. Some examples are the use of ozone with hydrogen peroxide, or ozone under UV irradiation, generating hydroxyl radical (OH⋅), the strongest oxidizing agent used in disinfection treatments.
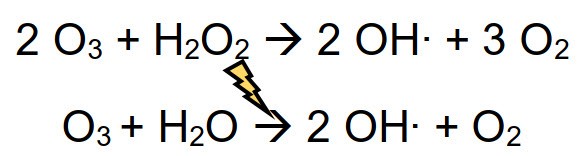
Radicals have a very short life-time (order of magnitude of milliseconds) and quickly react with almost any compound present in solution, through several mechanisms such as radical addition, hydrogen abstraction, electron transfer or radical combination. Radical paths are never selective and are divided in steps: activation, chain propagation and termination. Radicals involved in these reactions have a fundamental role in the disinfection process, because they are even stronger oxidant than their precursors (ozone, hydrogen peroxide, etc.)
One advantage of AOPs is the absence of harmful by-products, another is that they do not generate solid waste, which must be treated and disposed of, since radicals lead to the complete oxidation of organic compounds.
Non-oxidizing biocides
Non-oxidizing biocides (NOBs) are compounds that act against bacteria through several mechanisms: preventing duplication, stopping cellular processes and metabolic reactions or destroying the cell wall. The biocidal action is carried out through specific mechanisms that make NOBs characterized by a major tendency to induce resistance phenomenon. Non-oxidizing biocides do not act through redox reaction and, in general, they are less reactive than OBs - indeed, the time required to fully eliminate bacteria is longer (from several hours to one day). Being less reactive, NOBs can be stored for a very long period and they are less corrosive against materials. Another advantage of non-oxidizing biocides is the great stability in solution - they have low sensitivity to variations in pH and temperature, a prolonged effect and the biocidal activity is easy to control.
However, the stability of non-oxidizing biocides has also a negative counterpart, that is the persistence in solution and the risk of accumulation in wastewater. From the environmental point of view, this is of course a problem, because NOBs are in general toxic and harmful for the aquatic ecosystem.
Generally speaking, non-oxidizing biocides are molecules of moderate dimensions, as quaternary ammonium and phosphonium compounds (called QUATS), organic molecules with bromine and isothiazolinones. They are usually compatible with additives and other organic compounds presents in solution.
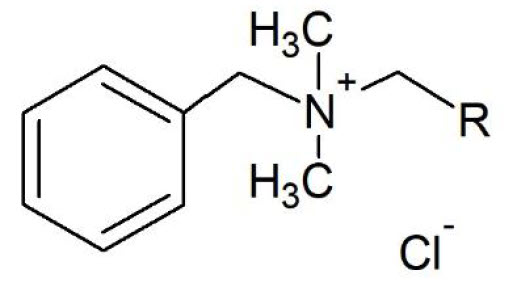
QUATS
Among the most widely known quaternary ammonium compounds, there are the alkyldimethylbenzylammonium chloride (ADBAC), with a variable length of alkyl chain, as in the following molecular structure.
Another similar compound is the didecylmethylammonium chloride (DDAC), which has a symmetric structure with two long alkyl chains, as shown below:
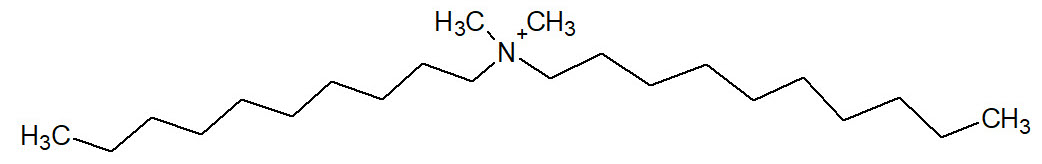
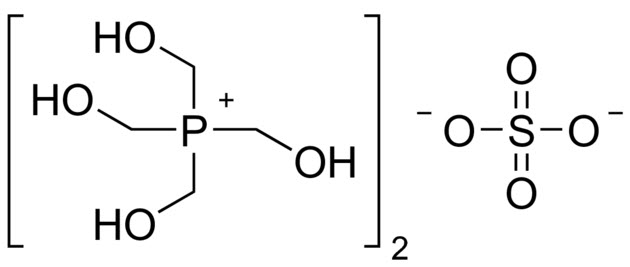
Among quaternary phosphonium compounds, the most famous is the tetrakis hydroxymethylphosphonium sulfate (THPS), especially used in the oil field.
QUATS are quite cheap cationic compounds, that are effective against a large number of microorganisms including algae, fungi and bacteria. They are active in a wide range of pH, but they have the tendency to develop foam at alkaline pH. The bactericidal activity is due to the formation of an electrostatic bond between the quaternary nitrogen and the proteins that constitute cell wall. When these proteins are denatured by the new bond, cell permeability is modified and so bacterial lysis occurs.
Unfortunately, QUATS are not effective against all the microorganisms - for example, they show a poor biocidal effect against Legionella.
PolyQUAT

There are polymeric materials with biocidal action formed by long QUATS chains, such as poly[oxyethylene (dimethyliminio)ethylene,dichloride], commercially called WSCP.
It is effective at all pH values, with the maximum activity in the range 7.5-9.0, and it combines a high antimicrobial strength with a very low toxicity and skin irritability. That is why WSCP is often used in applications as swimming pools and sanitary water systems, but also in cooling towers and in metal working. Moreover, it is compatible with almost all materials and it is usually applied in combination with oxidizing biocide, as chlorine, to reduce its dosage. Contact time is 6-8 hours and the mechanism of action is similar to that described for QUATS - an electrostatic bond between positive nitrogen and cell wall is formed, so the cell membrane breaks and leads to cell death.
BHAP

A non-oxidizing biocide particularly effective against biofilm is the 2-Bromo-4-hydroxyacetophenone (BHAP). It is used in applications where biocide is dosed continuously at low concentration (1-5 ppm), as the once-through cooling systems.
The biocidal activity of BHAP is pH-independent. Its stability, characterized by a half-life of approximately 200 hours, can be limiting because of the maximum concentration allowed in industrial wastewater.
Bronopol
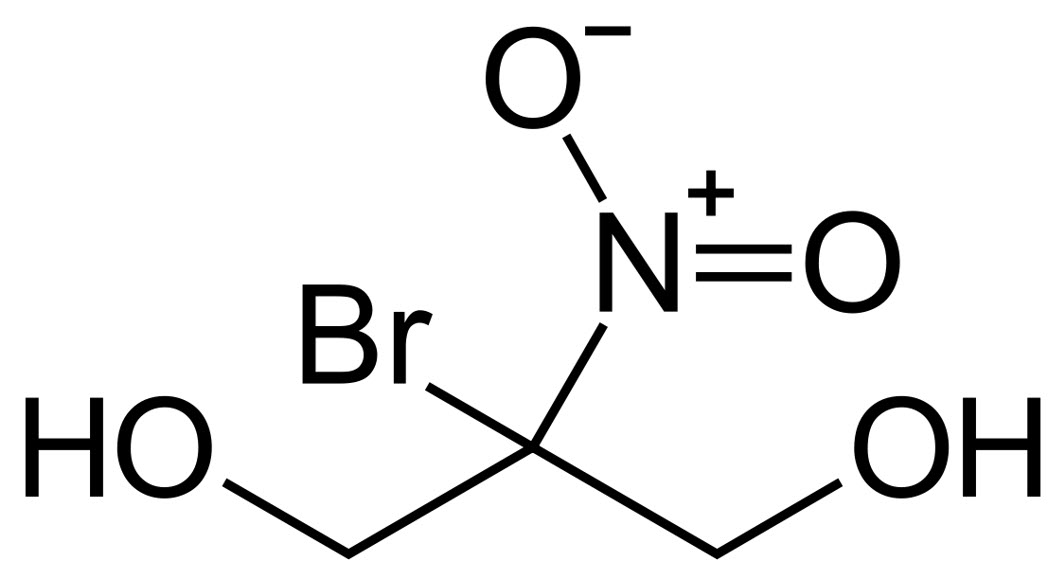
The 2-Bromo-2-nitropropane-1,3-diol, also called bronopol, is a diol with bromine substituent and a nitro group, as represented in the chemical structure. It is considered a general-purpose biocide, because it is effective against fungi, algae, aerobic and anaerobic microorganisms and it is compatible with all other non-oxidizing biocides. Bronopol works best at pH < 8.0
DBNPA
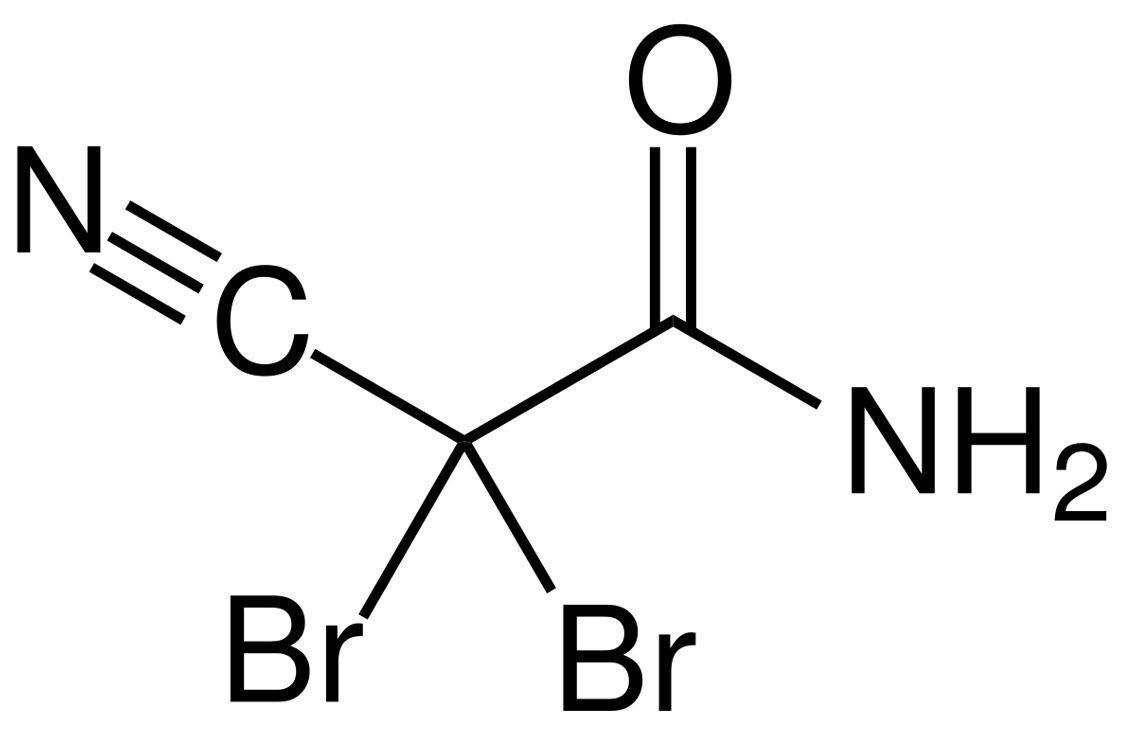
The most widely used non-oxidizing biocide is the 2-2-Dibromo-3-nitrilopropionamide (DBNPA), an amide with two bromine substituents and a nitrile group, as observed in the chemical structure. It is effective against biofilm and it has a good bactericidal action even where organic content is high. DBNPA is the NOB with the fastest action (contact time of about one hour) and it is often applied in combination with chlorine in once-through cooling systems.
As pH and temperature raise, DBNPA life-time rapidly decreases. So, it is not persistent and it fulfills the requirements in terms of concentration in wastewater. In alkaline conditions (pH > 8.0) the amide quickly hydrolyzes and releases ammonia. DBNPA is also degraded by visible light, but fortunately this is not a problem for water treatment.
The mechanism of action is oxidative, even if DBNPA is not an oxidant by itself, because bromine atoms are reduced by reaction with bacterial substrate. DBNPA also reacts with some cytoplasmatic components as thiol groups of proteins; this reaction results in the inhibition of cellular metabolism.
DBNPA can be added in liquid form (5% active solution) or as slow-release tablets (active up to 3 weeks).
DTEA
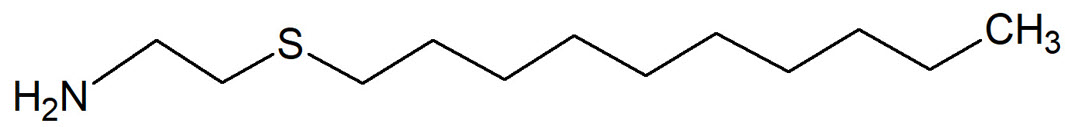
On the right is drawn the 2-(Decylthio)ethanamine (DTEA), a non-oxidizing biocide recently introduced on the market and designed to be effective against biofilm in a wide range of pH.
The DTEA forms reversible chelate complexes with inorganic ions present in the extracellular matrix of biofilm, whose structure is weakened and loses adherence to surface. DTEA is applied in disinfection treatments together with other biocides. Because of its bacteriostatic effect and of the mostly superficial activity, it is also defined a "biocidal soap".
Glutaraldehyde

Glutaraldehyde, an organic compound with two aldehydic groups as seen in the chemical structure, is a transparent liquid with strong pungent odor.
Glutaraldehyde has a strong biocidal action even against persistent microorganisms, thanks to the good capacity of penetration - however, it shall be used at high concentration (in the order of 100 mg/L). The action mechanism is the irreversible cross-linking with the lipoproteins of the cytoplasmatic membrane, that modifies permeability and leads to cell lysis. In addition, also the inactivation of periplasmatic enzymes contributes to the rapid death of bacterial cells.
Glutaraldehyde is quite reactive, and in acid environment it reacts with nitrogen compounds through addition reaction that leads to the formation of imines - so it gets disactivated as biocide. The speed of action and microbiocidal efficacy increases with pH, due to the larger penetration at alkaline pH. Thanks to its reactivity, glutaraldehyde has an excellent biodegradability - a very positive feature, considering that it is highly toxic for aquatic organisms - as well as a relatively short life-time (4-12 hours, depending on system conditions).
The main application is the disinfection of sanitary devices and instruments. Due to the quite high cost, it is not much applied in the industry.
Isothiazolinones

Isothiazolinones are a class of cyclic organic compounds, the most common are the chloromethylisothiazoline (CMIT) and the methylisothiazoline (MIT), usually mixed together in 3:1 ratio.
They act in a few minutes, deactivating the metabolism of microorganisms through interruption of transmembrane transport. However, the complete bactericidal action requires a much longer time (5-6 hours of contact). Isothiazolinones are effective against several bacterial species in the range of pH 6.5-9.0, while they have fungicide and algaecide effect only at acid pH.
Isothiazolinones are stable in water, so they are widely used as antimicrobials and stabilizers in cosmetics and detergents. It is important to pay attention to possible allergic reactions, because at high concentrations they are very irritating for skin and eyes.
Conclusions
It is clear that disinfection processes are fundamental, first of all for the safety and health of people, and then to ensure high hygiene standards aimed at limiting as much as possible bacterial contaminations. Secondly, sanitation treatments are important to limit microbiologically induced corrosion (MIC) and all the other issues related to bacterial proliferation in pipes, industrial plants and water networks in general.
Biocide treatments have a huge impact also from the economic point of view - in 2021 the global biocides market was estimated around 12.8 billion USD and in 2026 it is expected to reach 16.6 billion USD. The economic impact of biofilm, and associated phenomena, is also very high. The problems connected to biological corrosion have an annual cost of 2720 billion dollars and just in the marine sector biofilm causes 34 billion USD damages per year. The development of bacteria and biofilm growth are ubiquitous and affect all applications where liquids are present - wastewater treatment, cooling towers, potabilization, food industries, Oil&Gas, etc
To counteract biofilm, which includes 90% of the bacteria present in a system, the approach should be "prevent-detect-manage-engineer":
PREVENT: preventing the formation of biofilm is certainly the best choice to avoid problems caused by it later on, but unfortunately it is not always possible. However, its growth can be monitored to act as soon as possible, and to avoid that it becomes mature - it is much easier and cheaper to keep a clean system clean, than to get rid of dirt that has already accumulated.
DETECT: the ALVIM Sensor responds perfectly to the need for a biofilm detection device, allowing not only to monitor the formation of biofilm in real-time, starting from the first bacterial layer, but also to detect oxidizing biocides. So, this allows to verify the correct distribution of the biocide, to evaluate the real effectiveness of the applied treatment and, if necessary, to optimize it.
MANAGE: the choice of a sanitation treatment must be specific for each application, since the effectiveness of a biocide depends on many system variables. Some disinfection programs combine oxidizing and non-oxidizing biocides, to exploit the best of both. Other advanced methods, on the other hand, use the synergistic action of physical and chemical treatments. As reported in the summary scheme (Tab. below), oxidizing biocides can be extremely effective in destroying extracellular polymeric substances and, at the same time, bacterial cells. However, a sufficient residue shall be maintained, to completely oxidize biofilm. The main disadvantage of oxidizing biocides is their corrosivity towards metals, and the formation of disinfection by-products with a high environmental impact. Non-oxidizing biocides are generally much less effective against EPS, but they can penetrate deeply into biofilm and kill bacteria, weakening the structure of this biological layer, but not completely removing it. Using oxidizing and non-oxidizing biocides together is, therefore, an excellent strategy to optimize the performance of both types, reducing the overall dosage of chemical products.
ENGINEER: there are applications, such as wastewater treatment or bioreactors, where biofilm can play a positive role. Engineering biofilms that provide the best results, for the specific purposes of the application, represents a challenge that scientists and technicians are already facing all around the world.
|
Do you have biofilm-related issues?
|





