Residual Chlorine [Download this white paper as PDF file]
Chlorine-based chemicals are among the most widely used biocides for water systems. This is due both to their efficiency against pathogens, and to their large availability and low cost. Once dissolved in the liquid, chlorine species form strong oxidizing agents that chemically interact with the bacteria in the system, killing them. Hypochlorous acid and hypochlorite are strong biocides generated by chlorine dismutation in water. These biocides are more effective on free-floating bacteria, than on those attached to surfaces (biofilm). This is due to the exopolymeric substances (EPS) produced by bacteria within biofilm, that shelter them from external agents and treatments. Chlorine dioxide and chloramines are weaker biocides, however they are more stable and more effective in penetrating biofilm.

The biocide activity of these chemicals is linked to the biochemical interaction with the bacterial membrane. As a result of this reaction, chlorine species get consumed and bacteria get killed.
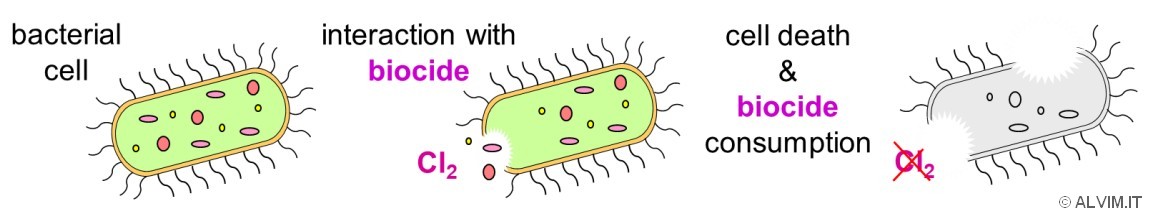
Chlorine-based biocides are usually dosed at a concentration high enough to leave a residual in water. This approach is intended as a safety measure, aimed at guaranteeing that all bacteria are killed. It is often assumed that measuring the value of residual chlorine, downstream to sanitation, provides a good indication both on the efficacy of the treatment and on the level of bacterial contamination of the system. Unfortunately, this is not correct. To make an example, if contact time is too short, or the chemical does not penetrate biofilm layer, there can be (even many) alive bacteria after treatment. Furthermore, many other chemical processes compete with bacteria removal for chlorine consumption.
The most common methods for measuring residual chlorine are based on colorimetric, amperometric, polarographic and gas-stripping techniques. These measurements can be performed on water samples or, more conveniently, directly online by means of different instruments. Depending on the analytical set-up, either total or free residual chlorine can be measured. Total residual chlorine is defined as the sum of free and combined residual chlorine. Free residual is mostly represented by hypochlorous acid and hypochlorite species. This residual includes species that are still highly reactive, and it indicates the excess oxidizing power deriving from chlorine dosage. Combined residual is composed of the sum of different chloramine species (mono-, di- and tri- chloramine). It indicates how much chlorine reacted with ammonia, nitrites and organic nitrogen (e.g. amino acids). Monochloramine is a considerably less effective biocide, compared to hypochlorous acid, while other chloramines are mostly ineffective. Noteworthy, chloramines interfere with free-chlorine measurement, causing the detection of ‘phantom’ free residuals. Other interferences affecting residual chlorine measurement are caused by oxidizing agents such as ozone, hydrogen peroxide and manganese compounds. Overall, residual chlorine measurement is very susceptible to interferences and errors.
Indeed, chlorine species are highly reactive both with organic and inorganic compounds, due to their strong oxidizing power (Fig. 3). The chemical consumption of these substances is due, for example, to their reaction with decaying vegetation, organic additives and mineral deposit (scale). In addition, these chemicals are also involved in the corrosion of metals and other structural elements of the system. The total consumption of chlorine species in this context is often referred to as the oxidizing demand of the system. The common misconception that such oxidizing demand is linked only to bacteria removal is the source of wrong approaches towards water treatment and sanitation.
As mentioned above, there are many chemical processes behind chlorine consumption. Moreover, chlorine species are often volatile, thus they can leave the system due to off-gassing.
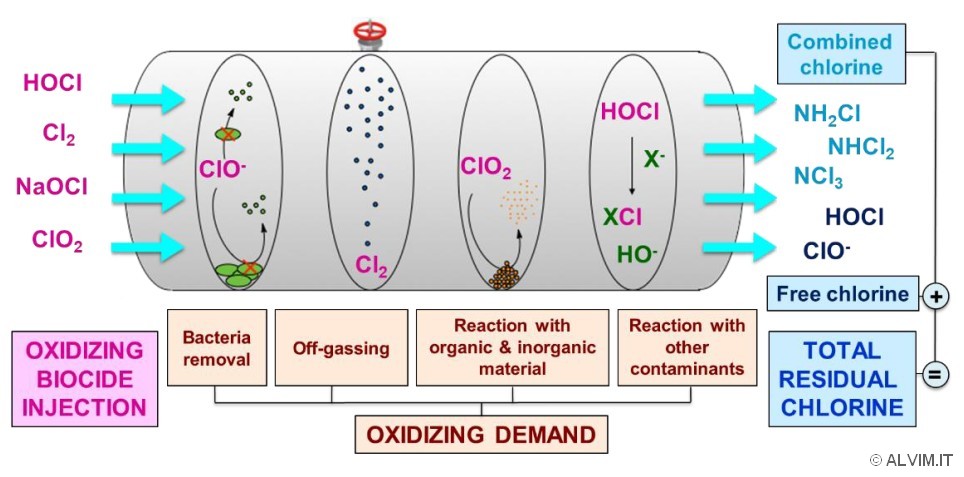
Thus, no detailed information on the causes of chlorine consumption is given by analysis of its residual. As a consequence, a simple measurement of residual chlorine cannot provide comprehensive information on the efficacy of biocide and, even less, on the amount of bacteria present in the system. A given oxidizing demand together with a given residual chlorine in a water system can result from different situations. Considering only these two parameters, it is not possible to assess treatment efficacy, understanding if biocide was under- or overdosed, and if most, many, or just a few bacteria were killed. Different problems might arise from wrong assumptions. For example, a given chlorine consumption, measured as residual chlorine, could be assumed as an indication of a clean system, where bacteria were removed. On the contrary, the partial removal of bacteria can be responsible for an increased amount of nutrients in the system, that might possibly lead to bacterial growth instead. Indeed, the destruction of microorganisms leads to the formation of readily available nutrients, thus increasing bacterial proliferation, even in the presence of biocides.
All these issues explain why the measurement of residual chlorine cannot be considered a reliable indication of cleaning and sanitation efficiency. The use of specific tools for the direct measurement of bacterial growth, such as the ALVIM Biofilm Monitoring Sensor, is strongly recommended instead. In a water system, more than 90% of bacteria live in biofilm, not free in the liquid. Moreover, this layer of bacteria is much more difficult to remove than free-floating microorganisms. ALVIM Sensor provides a reliable measurement of biofilm growth, on line and in real time. This allows to check the effectiveness of biocides, and to optimize the treatment based on the real needs.
|
Do you have a similar problem with biofilm?
|





