Biocides [Download this white paper as PDF file]
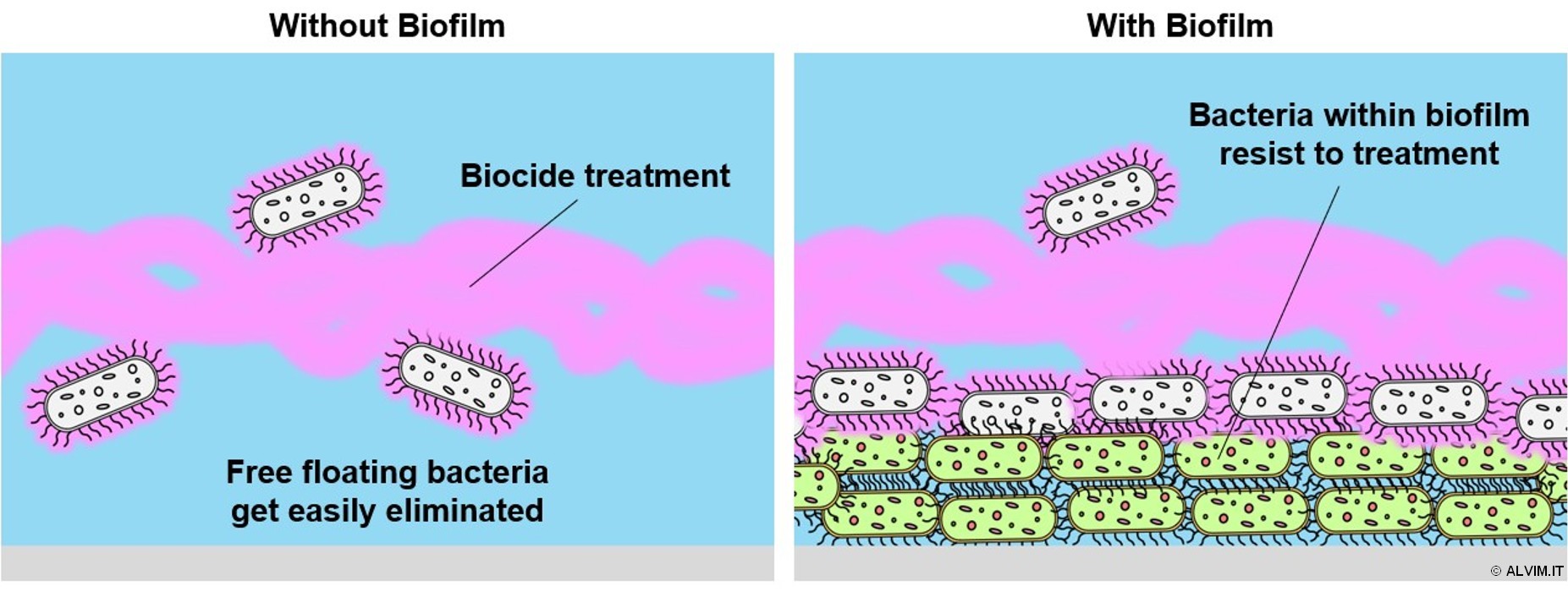
Harmful bacteria, viruses, and other pathogens proliferate in industrial and municipal water systems. Left unchecked, these microorganisms rapidly colonize all surfaces in contact with the liquid, giving rise to the phenomenon of biofilm. Within this slimy environment, bacteria and other pathogens find their ideal home. Indeed, 90% of total bacteria live in biofilms, and not free in the liquid. Therefore, this microbial layer is the main source of biological risk in water. As a result of biofilm growth on pipelines and other surfaces, a number of problems may arise, including corrosion, equipment failure, energy loss, reduced performances and increased energy consumption. In addition, biofilm growth leads to increased resistance to antimicrobial treatments. Indeed, the bacterial community living within biofilm is embedded in self-produced EPS (exopolymeric substances), which shelter it from the action of biocides. As a consequence, the eradication of biofilm is considerably more difficult, compared to the removal of free-floating bacteria (see figure below). On top of that, biofilm favors the proliferation of pathogens, such as Legionella pneumophila, Pseudomonas aeruginosa and Listeria, which can lead to severe outbreaks. No water system can be considered safe, as long as there is biofilm. For all of these reasons, it is highly important to get rid of this microbial slime. This can be done by treating the water system by means of an appropriate sanitation protocol, based on biocides.
Biocides represent chemical agents and formulations to control and eliminate harmful microorganisms. An appropriate biocide protocol is an important part of a municipal or industrial water system maintenance program. Both storage and application of biocides are highly regulated; the same applies to the environmental fate of both active and degradation products. In the US, the Environmental Protection Agency (EPA) regulates biocides as 'antimicrobial pesticides' according to the FIFRA (Federal Insecticide, Fungicide, and Rodenticide Act). The EU instead, has a different set of regulations, referred to as Biocidal Product Regulation (BPR). More generally, in all countries, biocide regulations are continuously subject to discussion, implementation and updates, as it was the case, in the year 2020, for Endocrine Disrupting Chemicals (EDCs).
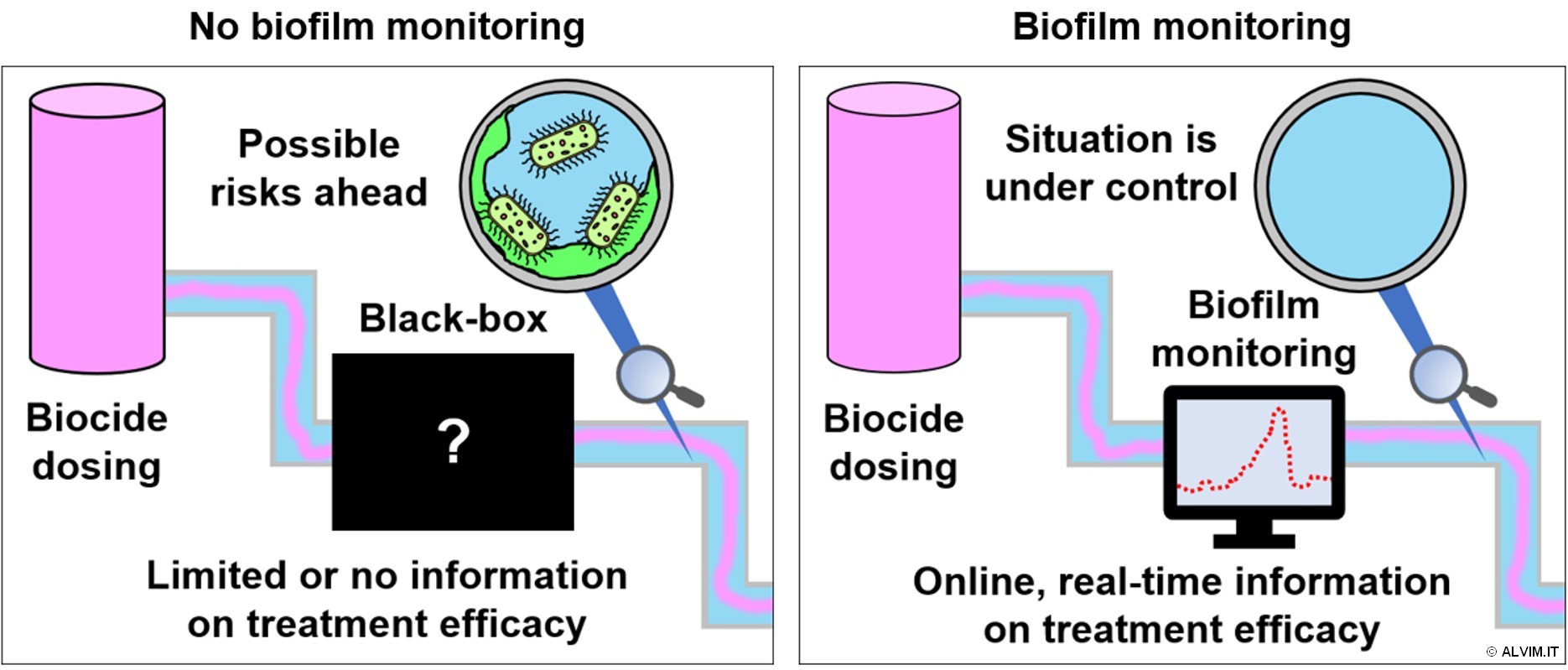
A very large number of biocides and related solutions are available on the market. There are many different parameters to be taken into account in the selection of a biocide treatment, depending on the specific application. Cost-effectiveness, system compatibility, dosage methods and times, related precautions and environmental outcomes, should be carefully evaluated in the selection process. Nonetheless, it is very important to note, that each water system is unique, and thus the efficacy of a biocide protocol cannot be exactly anticipated. With this respect, online, real-time biofilm monitoring is the best approach to assess the efficacy of a sanitation treatment (see figure below). ALVIM Biofilm Monitoring Technologies offer efficient solutions toward biocide protocol optimization, based on the real, specific needs.
Biocides are often categorized in two broad classes: oxidizing biocides (OBs) and non-oxidizing biocides (NOBs). The mechanisms of action, principles of application, monitoring methods, and responses to system contamination, differ significantly between the two groups. ALVIM Sensors are able to detect oxidizing compounds, including OBs, in addition to biofilm growth. Therefore, by means of this technology, it is also possible to conveniently monitor both biocide dosage and delivery, and thus to identify potential problems with the treatment.
Oxidizing biocides
Sanitation by means of OBs is the most employed solution to address microbial concerns in the field of water treatment. Chlorine and derivatives (hypochlorous acid, bleach, etc.), chlorine dioxide, bromine and derivatives (activated bromides, stabilized bromine chlorides and hypobromites, etc.), ozone, hydrogen peroxide and peracetic acid are the most commonly employed OBs. These compounds typically demonstrate a broad-spectrum activity across a wide range of microorganisms. OBs are typically fast acting; they feature multiple modes of action toward microbial deactivation. For this reason, they are not prone to induce microbial resistance. These compounds are highly effective even at relatively low concentrations. Such property makes them very cost-effective, which, together with their high effectiveness, explains why these chemicals are widely adopted in all industrial sectors. OBs are generally highly reactive. As a consequence, these compounds are very susceptible to the system demand: they interact with process contaminants, additives and organic matter. Indeed, high reactivity typically implies low selectivity; therefore, OBs get largely consumed by water systems. For this reason, these biocides are commonly employed in excess, in order to leave an active residual in the water and guarantee a persistent antimicrobial effect. Due to their oxidizing properties, OBs may induce corrosion phenomena in the systems where they are applied. For this reason, corrosion inhibitors are commonly integrated in the treatment to avoid this problem, but their interaction with OBs should be taken into account. Phosphonates, for example, which are largely employed as corrosion and scale inhibitors, chemically interact with most OBs, leading to the inactivation of both species. Moreover, OBs are generally sensitive to temperature and pH variations.
Chlorine and its derivatives
Water chlorination for disinfection purposes dates back to more than one century ago. As one of both the cheapest and the most effective chemicals available against waterborne pathogens, chlorine has been extensively used in the history of water treatment. Chlorine disinfection is not directly related to the action of the gaseous molecule, but rather to the products deriving from its hydrolysis reaction. Indeed, once dissolved in water, chlorine gas rapidly dismutates into hypochlorous acid (HClO) and hydrochloric acid (HCl).

Due to the increased acidity of the system deriving from the liberation of hydrochloric acid, chlorine is typically responsible of high corrosion rates. In addition, due to its high chemical reactivity, chlorine dosing leads to the generation of toxic Disinfection By-Products (DBPs) in water. These substances, which include tetrahalomethanes (THMs), haloacetic acids (HAAs), chlorophenols and other adsorbable organic halides (AOXs), are highly hazardous toward the environment, and thus their release should be avoided. In addition, chlorine species are volatile, and might decay due to off-gassing in piping systems. For this, and other reasons, including for example the strong safety measures required for gas storage and handling, molecular chlorine is poorly used nowadays.
Free chlorine
A number of methods able to deliver free chlorine directly in water are extensively used to replace gaseous chlorine dosage. The term free chlorine refers to the combination of two chemical species: hypochlorous acid and its conjugated base, the hypochlorite anion (OCl-). These two species constitute an acid-base couple; therefore, their concentration ratio in water is controlled by pH and temperature.

These conditions of the system influence not only the composition, but also the biocidal efficacy of free chlorine. Indeed, most industrial water environments settle around neutral to alkaline pH levels. In these conditions, the hypochlorite anion, which is up to 100 times less effective with respect to its conjugated acid, is the favored species. Chemicals dissolved in water, present as contaminants, or employed as process additives, also influence the disinfection properties of free chlorine. Both hypochlorous acid and the hypochlorite anion readily react with ammonia and other nitrogen compounds, leading to the formation of chloramines. Mono-, di- and tri- chloramines are referred to as combined chlorine. The general biocidal efficacy of combined chlorine is orders of magnitude lower with respect to free chlorine. Nonetheless, monochloramine (the major component of combined chlorine in common conditions) is employed as a biocide, due to its increased ability to penetrate microbial biofilms, as opposed to free chlorine, that has a poor effect instead. Many water distribution networks (especially those located in the US) make use of chloramines as a disinfectant throughout the distribution system. This biocide formulation, usually achieved by adding ammonia to free chlorine, maintains a long-lasting residual. In addition, by reducing the interaction of free chlorine with organic matter, it leads to a lower formation of DBPs. Despite free chlorine delivering methods are extensively more employed, compared to those based on combined chlorine, the residual contribution of both to water composition is commonly monitored as total residual chlorine. More detailed information on total residual chlorine, its relation with biofilm growth, and with disinfection procedures, can be found in another white paper by ALVIM.
There are many different approaches toward water disinfection by means of free chlorine. Commonly, a concentrated bulk solution of bleach is diluted and delivered in the system. This method is simple and efficient, but the high concentration of the solution might lead to some disadvantages, such as compromised accuracy of feed-pumps and increased decomposition rate of the active ingredients. These problems might be avoided with the use of solid formulations, which commonly consist of calcium hypochlorite tablets. Although this approach guarantees longer shelf life and stability, it also requires dedicated equipment for both preparation and stirring of the solution before dosage. A more advanced approach consists in the 'On Site Hypochlorite Generation' (OSHG) system, which allows to generate free chlorine, starting from simple raw materials such as sodium chloride, by electrolysis. In this case, the biocide solution is readily dosed in the system, thus preventing any decomposition phenomena or safety issue. Based on the same principle there are also techniques referred to as 'Mixed Oxidant Solutions' (MOS), which may address special needs, such as improved biofilm penetration, by generating more complex and potent formulations. Both approaches come with the inconvenience of generation of hazardous hydrogen gas, as a byproduct. In some other applications, a stabilizer, which is typically a small organic compound, is added to the formulation of free chlorine, in order to enhance its biocidal properties. By this method, it is possible to increase the stability of the active species and thus guarantee better performances, including biofilm removal. Indeed, many biocides, including free-chlorine, cannot efficiently penetrate biofilm because they get prematurely consumed upon reaction with the EPS layer. On the contrary, the stabilized forms of free chlorine are stable enough to penetrate the microbial layer and kill bacteria living inside (see figure below). Although this system allows to reduce and optimize the use of free chlorine, some environmental concern might be connected with the disposal of these stabilizers.

Chlorine dioxide
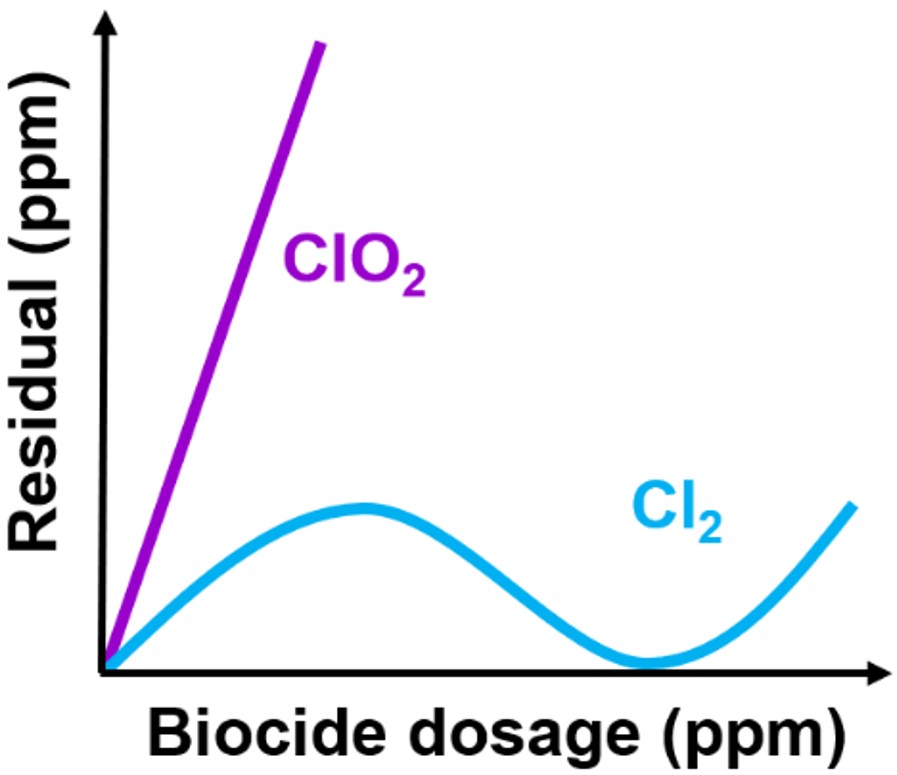
Chlorine dioxide is a gas like molecular chlorine, but it does not undergo any hydrolysis reaction in water, where it is highly soluble instead. This chemical property constitutes a great advantage because, unlike chlorine, this biocide is stable in a wide range of pH values (3÷10). In addition, due to its very diversified modes of action, microbes do not develop resistance against it. Although potentially dangerous and explosive at high concentrations, chlorine dioxide is safe to use at the relatively low concentrations applied in water treatment. In this context, chlorine dioxide is useful for many applications other than disinfection, ranging from odor control to destruction of contaminants. With this regard, it is similar to chlorine, because it gets involved in several chemical reactions. Nonetheless, chlorine dioxide has a lower and more controlled reactivity and, thus, a higher selectivity, compared to free chlorine. For this reason, it is more effective, and capable to leave more active residuals, while dosed at lower concentrations (see figure on the right). While chlorine dioxide is a more selective biocide, it is still affected by the chemical species which give rise to the 'oxidizing demand' of the water system. Indeed, many compounds commonly present in water systems can react with chlorine dioxide, leading to its chemical consumption. For this reason, it is strongly recommended to check the real efficacy of this (and other) disinfection treatment, e.g. applying biofilm monitoring. Apart from increased chemical selectivity, chlorine dioxide is characterized by three other advantages. First, it shows better performances in terms of biofilm removal, compared to other OBs. Since the interaction with EPS does not consume it, this biocide can efficiently penetrate microbial layers and kill bacteria. Secondly, chlorine dioxide leads to reduced corrosion issues compared to free chlorine, due to its lower oxidation potential (and thus lower reactivity toward metals). Last but not least, this chemical species does not form chlorinated toxic DBPs (as it happens with chlorine). As a consequence, its application has a reduced environmental impact, compared to other chemicals employed in water treatment.
Due to its instability in pure form, chlorine dioxide often needs to be generated on site; as a consequence, its utilization requires to adopt expensive equipment. More recently, chlorine dioxide generating formulations hit the market in the form of tablets, but these come with a poor chemical yield and poor stability. In addition, both the reagents (chlorites and chlorates) and the active product are hazardous for human health, so they shall be managed carefully. Moreover, as a very volatile gaseous compound, chlorine dioxide might leave the system due to off-gassing, when the system is aerated (e.g. in open cooling towers).
Bromine and derivatives
Unlike chlorine, molecular bromine is not offered as a biocide as such, due to its higher toxicity and reactivity. In order to maintain disinfection residuals, high concentrations of bromine should be employed. For these reasons, bromine derivatives do not find applications in drinking water systems or Food&Beverage production plants. Nonetheless, a number of formulations and delivery systems have been developed to provide convenient access to hypobromous acid (HOBr). The latter is a very active biocide, employed in many industrial environments.

Note: the higher is the pKa of an acidic compound, the lower is its tendency toward dissociation in water
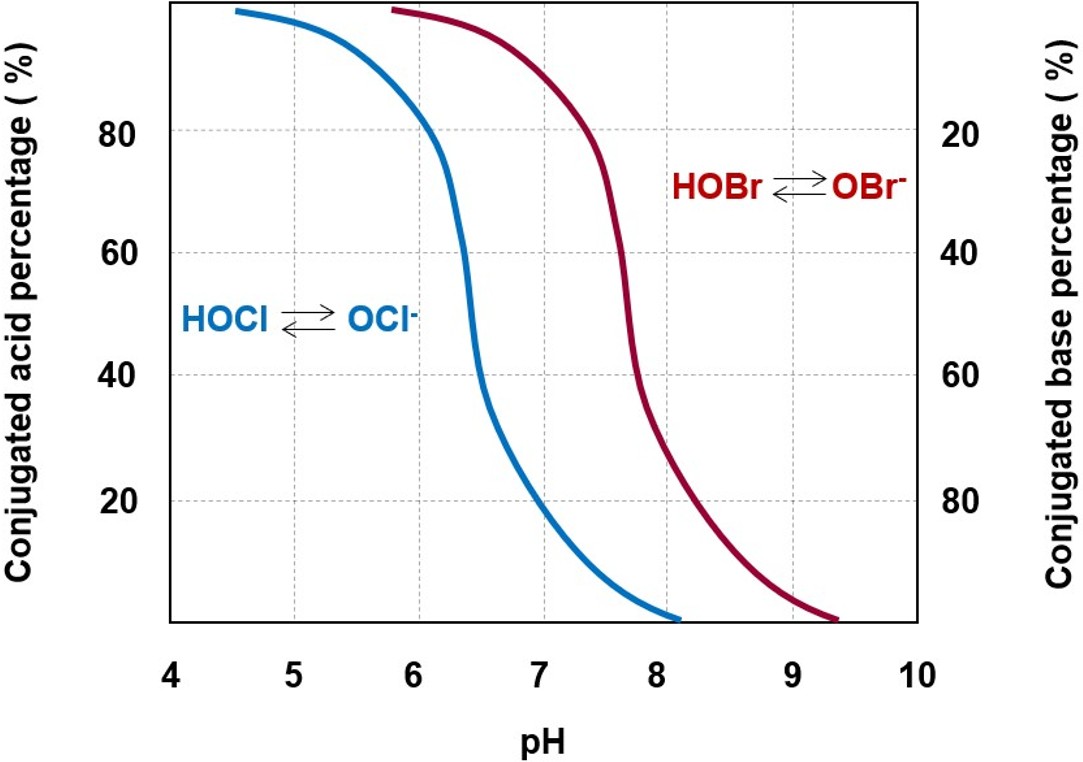
As a weaker acid with respect to hypochlorous acid, its brominated counterpart has a lower tendency to dissociate into its conjugated base, the hypobromite anion (OBr-), the brominated counterpart to the hypochlorite anion (see figure on the left). As a consequence, hypobromous acid is generally the most abundant chemical compound constituting free bromine residual composition. For this reason, bromine-based biocidal formulations perform better than their chlorine counterparts, especially at moderately alkaline pH levels. Bromine chemistry is similar to that of chlorine, since it is characterized by high reactivity toward a wide range of compounds that might be found in solution. Due to their high reactivity, bromine derivatives are considerably more effective toward planktonic bacteria, rather than biofilms.
Free and combined bromine species are generated and monitored in much the same way as for chlorine. No accurate analysis is needed to distinguish among the two, since combined bromine species are still as effective biocidal compounds as hypobromous acid. Free bromine is delivered in water systems mainly by two approaches: activation of bromides or stabilization of bromine chlorides and hypobromites. In the first case, free bromine is achieved by the interaction of a sodium bromide feed solution, with an activating agent, like bleach or ozone (mixing), or via the application of a direct energy source (electrolysis). In the second case, stable bromine derivatives are directly dosed in solution. In this way, the typical high, quick reactivity of free halides is avoided, favoring a more stable, slower effect. Bromine chloride for example, has better stability, storage and handling properties with respect to bleach formulations. The same is true for hypobromite species, which can be released in solution by stable organic derivatives. Such compounds exploit nitrogen based functional groups to bind (and stabilize) active bromine atoms.
Ozone
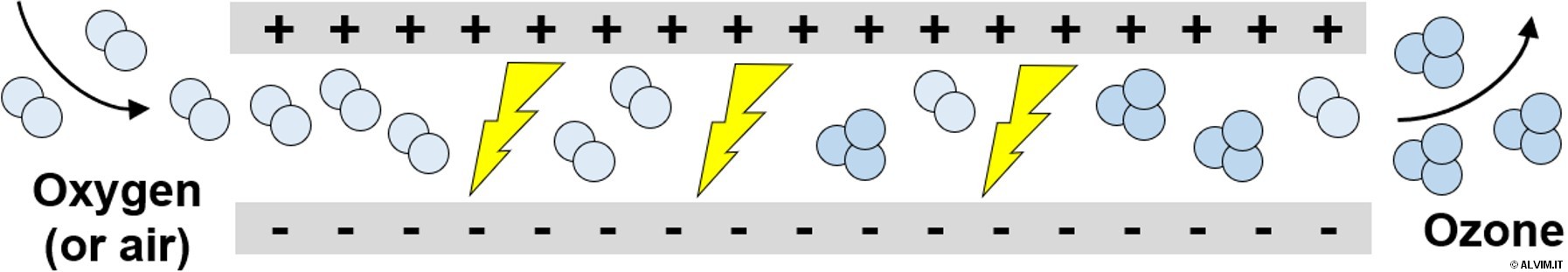
Ozone is an unstable gas, which is quickly decomposed in water (with a half-time of 5-20 minutes). Nonetheless, it is able to exert a very potent biocidal effect on all kinds of organisms, within a very short contact time, due to its high oxidizing power. In addition to its lack of stability, ozone is also only partially soluble in water. Similarly to chlorine dioxide, ozone easily abandons the solution due to its volatile nature. For these reasons, it is generally difficult to establish an ozone active residual in water. In order to guarantee the complete sanitation of the system, it is often necessary to perform multiple injections in different points of the system. As a strong oxidant, ozone is very aggressive towards additives and chemicals employed in water treatment, and highly corrosive toward metals and other materials constituting pipelines, valves, seals, etc. As it was the case for halides, ozone is too reactive toward EPS to penetrate biofilm efficiently. On the other hand, unlike halides, it does not leave any DBPs in the system. For this reason, ozone is highly suitable for Food&Beverage applications and, at the same time, more environmentally friendly than other OBs.
Ozone needs to be generated on-site, prior to use, by means of UV irradiation or corona discharge methods (with the latter being the safest and most widely adopted option in industrial applications). Dry oxygen or air need to be exposed to a high voltage or high frequency electrical field in order to generate ozone (see figure on the right). As a consequence, expensive generators able to remove heat, along with dedicated precautions, are required to prevent any hazard. Ozone is often employed in combination with UV treatment in applications referred to as 'Advanced Oxidation Processes' (AOPs). Via these methods, short-lived chemical species of even higher oxidizing power, such as the hydroxyl radical, are generated to achieve improved biocidal efficiency.
Hydrogen Peroxide and Peracetic Acid
Hydrogen peroxide (H2O2) is extensively used as a biocide, particularly in hygienic applications. This is because similarly to ozone, the decomposition of hydrogen peroxide does not lead to the formation of toxic DBPs. For this reason, this biocide is especially employed in drinking water, Food& Beverage and related applications. Despite a good ability to penetrate biofilms, and a good effectiveness at all pH ranges, hydrogen peroxide is characterized by a lower biocidal power with respect to other OBs. As a consequence, it is not extensively used as a main biocidal compound. On the other hand, it finds applications in combination with other chemicals, in order to enhance their effectiveness, and reduce the production of undesirable DBPs.
Hydrogen peroxide can be delivered via dilution of concentrated solutions or generated on-site by means of electrolytic methods. Upon blending of hydrogen peroxide with acetic acid, in the presence of acidic catalysts, peracetic acid (PAA), a more efficient biocidal compound, can be achieved. While PAA is also a highly hygienic compound, its efficacy is considerably impacted by alkaline media, becoming progressively less effective at high pH values.
Non-Oxidizing Biocides
NOBs typically consist, with some exceptions, of small organic molecules. As opposed to OBs, these compounds are usually highly compatible with any process additives and organic matter which might be present in water. Indeed, NOBs are generally less reactive chemical species, compared to OBs. While the former require hours to be effective, the latter are fully active in the order of minutes. Indeed, NOBs are characterized by a long shelf-life (typically 1 year or more). Their low reactivity is highly beneficial in terms of preservation of materials because, at use levels, they are considerably less corrosive than OBs. The biocidal activity of NOBs is highly controllable: since they are very stable in solution, they guarantee a long-lasting residual effect. In addition, NOBs are only slightly influenced by typical variations of pH and temperatures across industrial water systems. On the other hand, being persistent substances in water, they sometimes lead to increased concerns for effluent discharge. This is particularly important, considering that these substances are generally more environmentally toxic with respect to OBs. Due to their specific mechanisms of action, NOBs are more likely to induce antimicrobial resistance. Last but not least, these biocides are generally more expensive, compared to OBs.
A great variety of NOBs are exploited in the field of industrial water treatment. Among the most commonly and active ones there are: quaternary ammonium and phosphonium compounds (usually referred to as QUATS), organobromine derivatives, isothiazolones and glutaraldehyde.
The reference QUATS compounds are Alkyldimethylbenzyl- and didecyldimethyl- ammonium chlorides (ADBAC and DDAC); more branched derivatives are finding a large number of applications. These cationic compounds show a broad spectrum efficacy versus a number of microorganisms, but they are reported as only poorly effective against Legionella. Phosphonium QUATS, such as Tetrakis Hydroxymethyl-Phosphonium Sulfate (THPS), find relevant applications in many industrial sectors, including Oil&Gas.
Among organobromine derivatives, 2,2-dibromo-3-nitrilopropionammide (DBNPA) is the most employed one. As a quick-killing bactericide, it is not persistent, and it is rapidly degraded in nontoxic compounds via hydrolysis.
Isothiazolones (also referred to as isothiazolinones or isothiazolines) are water stable, slower killing biocides, capable of inactivating the microbial metabolism within minutes. Chloromethyl- and methyl- isothiazolinones (CMIT and MIT) are the most widely adopted, usually employed together in a 3:1 mixture. These compounds are broadly effective and compatible with many additives, with the exceptions of reducing agents and concentrated, strong oxidizers.
Glutaraldehyde is a potent, readily biodegradable biocide, used in various treatments. To be effective, it has to be used in a relatively high concentration (up to 100 ppm). While it is usually employed across a wide range of pH, it suffers the drawback of getting deactived by ammonia and other nitrogen compounds.
Dispersants and Enzymes
Dispersants, or biopenetrants, are non-biocidal, surface-active agents that penetrate and loosen the deposit formed on the surface of pipes, including the biopolymeric matrix. By these means, the microorganisms living within biofilms loose the EPS shelter and become more exposed to the action of biocides. As a consequence, bacteria can be eliminated with higher efficiency. For this reason, dispersants are often added to biocide formulations to improve their ability to remove biofilm. Additionally, their use allows to reduce the amount of biocidal products required to fully clean the system, resulting both in considerable savings and in a lower toxicity of effluents. Dispersants are typically dosed in the system prior to biocide. A wide variety of cationic and anionic dispersants are available on the market. Recent applications instead involve the use of nonionic polymers, which are non-toxic and non-foaming. With this respect, enzymes are very promising too, and they are receiving increasing attention in the field of water treatment. As it is the case for all dispersants, they shall be always coupled with biocides. Otherwise, breaking biofilms without eliminating the bacteria living inside it, would result in a large increase in the number of microorganisms - and, possibly, of pathogens - in the water (see figure below).
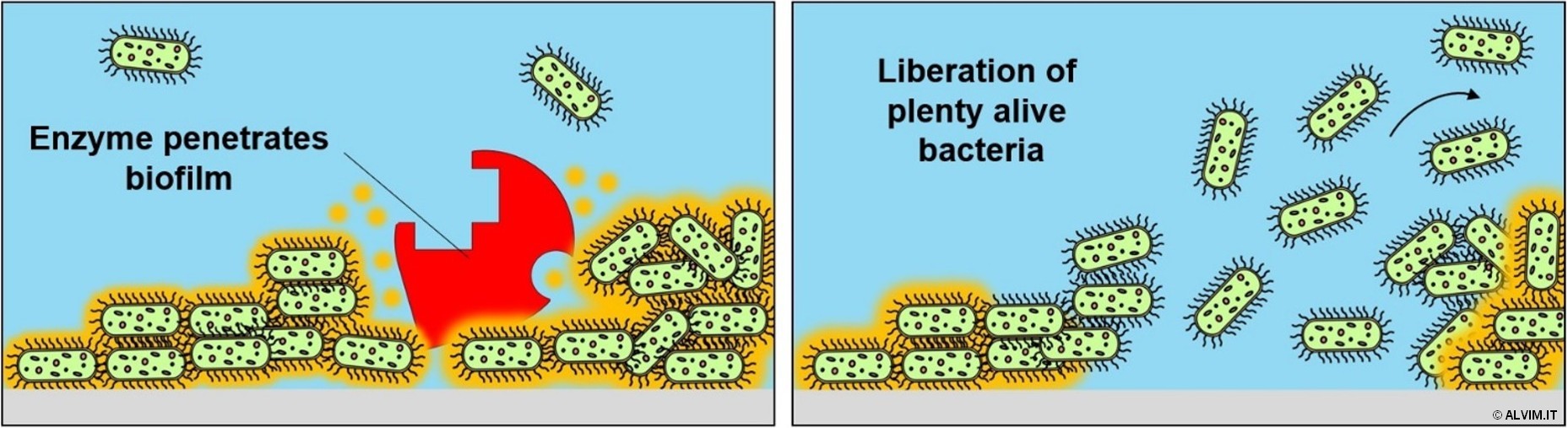
Enzymes are sensitive towards oxidizing chemicals, which might denature them. Since biocides are usually dosed after enzymatic treatment, in most cases this is not a problem. However, due to the cost, the use of enzymes is currently limited to Food&Beverage and some other applications where the volumes of water are not too large.
As a concluding remark, let us underline that the selection of a suitable biocide for a specific application is not sufficient to guarantee optimal results against bacteria. In order to keep pipelines clean, prevent problems, save money and time, it is necessary to check the real efficiency and to optimize the treatment. ALVIM offers the best technologies to assess the efficacy of sanitation treatments, based on real needs.
|
Do you have biofilm-related issues?
|





