Biofouling in membrane processes [Download this white paper as PDF file]
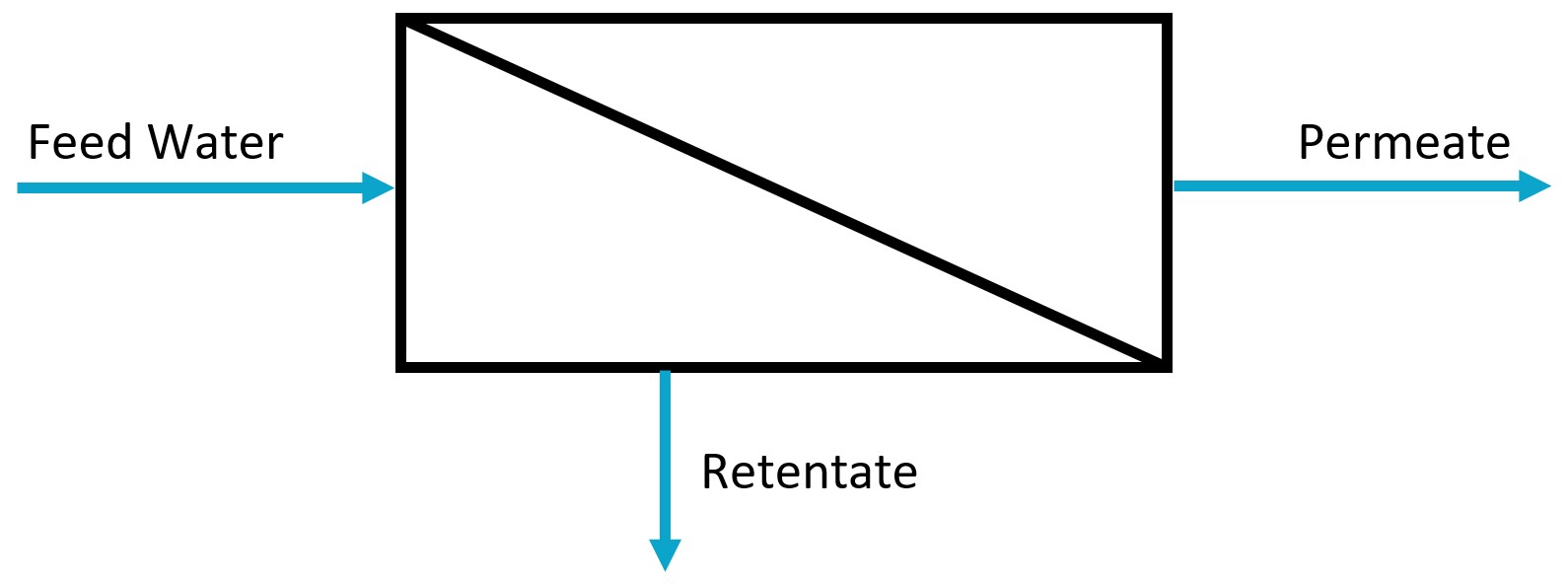
Membrane processes cover a group of separation methods where the components of a solution or a suspension are separated by means of a membrane. The feed stream is split into two fractions (see figure on the right), the one that permeates through the membrane (permeate), and the one containing the components that have not been allowed through the membrane (retentate). Membrane processes are more and more applied in water and wastewater treatment due to their reduced volume, and high separation efficiency, compared to traditional methods. Characteristics of the membrane, such as porosity, selectivity, electric charge, are essential for the separation.
Membrane processes may be classified depending on the size of the components to be parted or on the working pressure (see table below). Membrane systems can operate either with constant permeate flux and variable transmembrane pressure (TMP) or with constant TMP and variable permeate flux.

A major obstacle for the application of membrane is the loss of efficiency over time, due to fouling. Four categories of foulant can be recognized: particulates, which physically block the pores; organic material, which attach to the membrane by adsorption; inorganic components, which precipitate on membrane surface; microorganism, such as algae and bacteria, which adhere to the membrane blocking the pores, forming the so-called “biofouling” or “microfouling”. When membrane fouling occurs, permeate flux will decline if working at fixed TMP, or feed pressure will increase if working at fixed permeate flux (which is the most common approach). Thus, productivity decreases with the increase in membrane maintenance and operation costs due to cleaning. Membrane fouling and efficiency are the main challenges for membranes related technologies. As mentioned above, biofouling represents an undesirable accumulation of microorganisms which occurs by attachment, growth and metabolism of bacteria on the membranes. There are two main components of microfouling, bacterial cells and extracellular polymeric substances (EPS), produced by the microorganisms. In many applications, the EPS fraction has been found to be the largest part, accounted for 50–80% of the total organic matter. Among the various filtration processes listed in Tab. 1, biofouling is of major concern in industrial nanofiltration and reverse osmosis applications. These techniques are commonly used for water softening, drinking water production, process water production, nitrates removal and ultrapure water production. Many treatments are applied to control membrane fouling, biological or not. In this paper, an overview of the different approaches to biofouling monitoring and control in industrial NF and RO membrane applications will be provided. Commonly, the development of a fouling layer is detected indirectly by measuring the loss in permeate flux or the increase in trans membrane pressure. These analyses are cheap and easy to perform and also the outcomes, based on Key Performance Indicators (KPIs), are simple to understand for the operator, to recognize the necessity of a cleaning treatment. Usually, when the TMP drop reaches 5-10% the cleaning is triggered. However, at such values of pressure drop the fouling layer may already be thick and well established, therefore difficult to eradicate. Also, there is no way to distinguish the kind of fouling - organic, inorganic, biological, etc. - but, in order to choose the appropriate cleaning treatment, it is necessary to know if the detected fouling is completely, partially or not at all biological.
Ultrasonic time-domain reflectometry (UTDR) is getting more and more popular for online and real time biofouling monitoring. When ultrasonic waves reflect on the interfaces (such as water/membrane interface, water/fouling interface), the thickness and identity of the fouling layer can be determined through the return time and the magnitude of the waves. Even if UTDR technique has been applied to many different membrane separation processes, it should be pointed out that, since the acoustic properties at the different interfaces only slightly differ, it is not easy to distinguish biofouling from other kinds of fouling. Electrical impedance spectroscopy (EIS) is also receiving an increasing interest, to monitor in a non-invasive way the membrane fouling, based on the fact that, when this layer starts to accumulate, the electrical properties of the membrane change. Compared to conventional fouling measurement, such as permeate decline and transmembrane pressure increase, EIS is much more sensitive and has the potential to distinguish among different kinds of membrane fouling. While EIS has been proved to be an effective method, its suitability for industrial applications still needs to be tested and adjusted in the field. Another available option is to inject a fluorogenic agent in the feed water. As it interacts with microorganisms, it will trigger a change in the fluorescent signal which is then detected by an on-line fluorometer. The fluorogenic agent shall not reduce the efficacy of chemical pretreatment and should be compatible with membrane materials, other than being environmentally friendly.
All the previously named analyses allow for an online fouling detection, but many of them do not distinguish the kind of fouling or may return an alarm only when a thick layer has already developed, or may require the use of chemicals that are not compatible with the process. It is clear that a specific tool for online biofouling detection is still to be found.
The first step for the control of biofilm development takes place during the project phase, where hydrodynamic conditions and design are determined, together with membrane material and properties. Membrane fouling can be limited by increasing either the shear rate or turbulence near membrane surface. However, increasing turbulence would increase nutrient supply to biofilm. Optimization of hydrodynamic conditions is essential for preventing or at least minimizing biofouling. The degree of biofouling adhesion depends on the properties of microorganisms, solution and surface. Membrane fouling is also closely related to surface properties, such as smoothness and hydrophilicity - smooth and hydrophilic surfaces show less fouling tendency. These qualities can be enhanced, and so the fouling resistance, by surface modification, such as surface coating (SC), a physical modification, and surface grafting (SG), a chemical modification.
For sure, an optimal design helps to reduce biofouling, but it is not enough. Therefore, further actions are required. Usually, as soon as TMP increases it is assumed that the system needs to be cleaned. Membrane cleaning methods are divided into physical and chemical; the former includes hydraulic (such as flushing and backwashing), pneumatic and mechanical processes, while the latter involves the use of chemicals (acids, bases, oxidants and surfactants). Usually, physical cleaning followed by chemical treatment is widely employed for maximizing the effect. However, if there is much biomass in the feed channel, the transport of chemicals can be limited, making hard to completely remove biofouling. For this reason, early cleaning of partially fouled systems is crucial. Usually, chlorine-based biocides are applied for pretreatment, due to their efficacy toward general bacteria. Since most commercially available polymeric membranes are sensitive to oxidizing compounds, chlorine shall be neutralized dosing sodium bisulfite, before treated water reaches the membrane. Ozone is also widely applied as a disinfectant in water treatment, due to its strong oxidative effects. However, because of its instability, ozone has to be produced on-site. Also in this case, direct contact with polymeric membranes shall be avoided. Non-oxidizing biocides such as formaldehyde, glutaraldehyde and quaternary ammonium may also be applied as they are less aggressive towards membranes, but after a while bacteria could become resistant (as discussed in this white paper). Biocides are often dosed continuously, leading to possible environmental, ecological and toxicological problems, and increasing treatment cost. Specific biological monitoring should be conducted, to apply chemical treatments only when needed. Other applied pretreatments involve the use of coagulant and flocculant to aggregate the microbial cells and make them suitable for sedimentation before the filtration step. Some recent studies compared the traditional use of coagulant and flocculant with the application of a coarser filtration step. For instance, if RO is applied for water desalination, then MF and UF may be used to pretreat feedwater. In this way the amount of chemicals is cut down and process efficiency increases, while reducing the whole process volumes - indeed, membrane units are more compact than the tanks used for coagulation and flocculation.
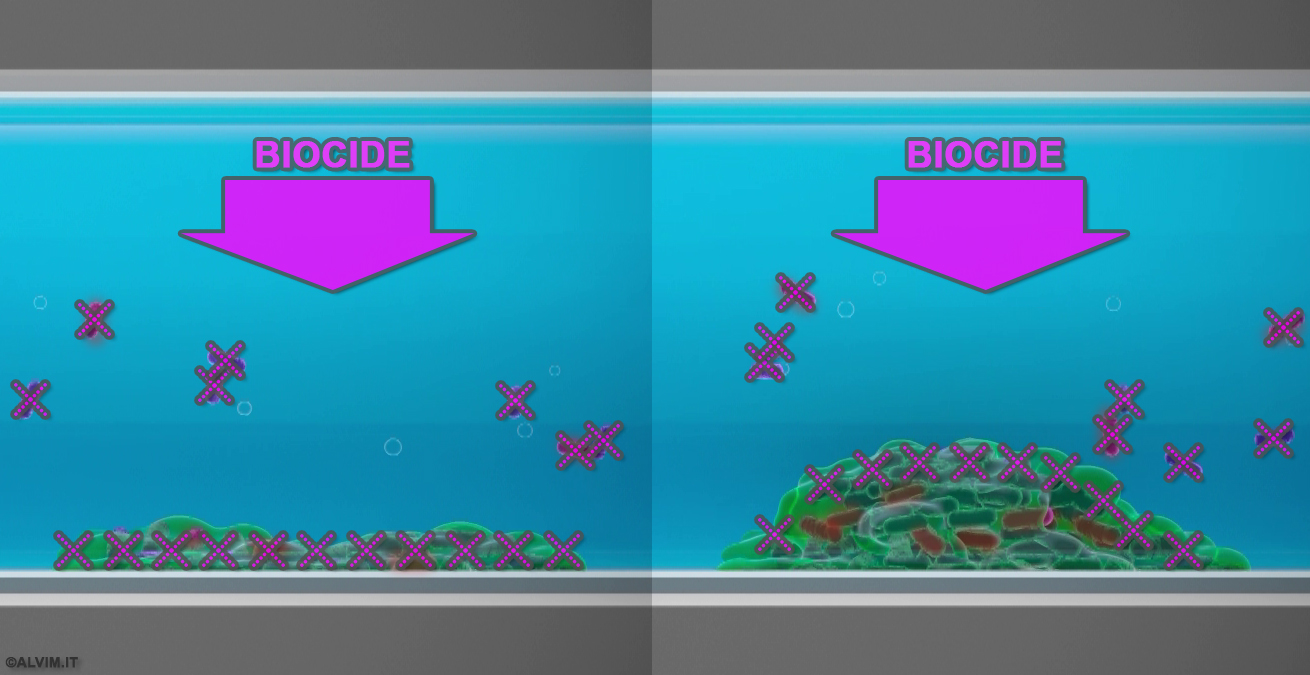
EPS secreted by bacteria ease bacterial attachment to membranes and act as a shelter to protect the colony from chemicals (see figure below). The physical and chemical treatments mentioned above are not always able to efficiently remove EPS, while these can be hydrolyzed by some specific enzymes. On the other hand, it shall be considered that the activity of enzymes can be largely reduced if they are not applied at optimal pH. Moreover, enzymes are highly sensitive to temperature - high temperature may denature enzymes, whereas low temperature can decrease the enzymatic activity significantly. Still, compared to the chemical methods for EPS removal, the enzymatic approach is nontoxic and environmentally friendly, while the problem associated with the bacterial resistance to antifouling agents is minimized.
Among the most recent techniques, currently applied only in lab or pilots, silver nanoparticles (Ag-NPs) show a high effectiveness in controlling the growth and activity of various planktonic microorganisms and, though they are not effective against mature biofilm, they may be used for delaying biofilm formation. This pretreatment method is not widely employed in industrial membrane systems, as it may not be a cost-effective choice at an industrial scale, due to the large treated volumes.
Microorganisms can coordinate their behaviors (e.g. biofilm formation) thanks to quorum sensing (QS). The QS-coordinated process is reached by producing, releasing, and detecting the autoinducers (AIs), small signal molecules. Quorum sensing could represent the key switch from the behaviors typical of single planktonic cell to a colony behavior. Disruption or inhibition of QS systems seems like a promising method for controlling microbial attachment, and consequently membrane biofouling. So far, quorum sensing inhibitors have been used mostly in laboratory application with pure cultures, therefore further study is required for biofouling control in industrial-scale membrane systems.
It appears clear that a good process and material design is necessary to mitigate membrane biofouling and increase system performances. Still, this it is not sufficient and other actions are required to control biofilm growth. Chemical dosing is, up to now, the most common technique. But usually, biocide dosage is performed without knowing the real need of the process, as none of the mentioned techniques allow precise and specific biofouling monitoring, in industrial applications. ALVIM first-in-class technologies for biofilm monitoring represent a powerful tool for detecting bacterial growth on surfaces in contact with liquids. Thanks to the data provided by the ALVIM System, on line and in real-time, it is possible to manually or automatically adjust and optimize biocide treatments, checking at the same time the efficacy. In membranes systems, ALVIM probe can be applied before the membrane to monitor biofilm growth in pipelines and tanks and, knowing microbial activity, to dose chemicals based on real needs (here an example related to biofilm detection in Seawater Reverse Osmosis Desalination - SWRO, and here another example regarding biofilm monitoring in industrial reverse osmosis). In this way, also sodium bisulphite feed can be optimized, where it is applied to neutralize chlorine. This provides several advantages:
- increase in process efficiency;
- increase in membrane lifetime;
- reduction in downtime;
- reduction in operational costs;
- reduction in environmental impact.
|
Do you have biofilm-related issues?
|





