Bacteria Detection [Download this white paper as PDF file]
Microbial control practices are applied in all industrial and civil applications where water or other liquids are employed. Despite its critical importance, the approach to this topic is often incomplete, or even wrong. Commonly, several efforts are spent to monitor free-floating bacteria in the liquid. Even if this is a widely-applied standard, it does not keep into account the layer of bacteria attached to surfaces in contact with the liquid, which is usually known as biofilm. Up to 90% of total bacteria present in a piping system live in this bacterial slime, and the analysis of water/liquid samples cannot detect them. For this reason, this standard method provides only a partial, limited view of the microbial contamination of the system (see figure below).
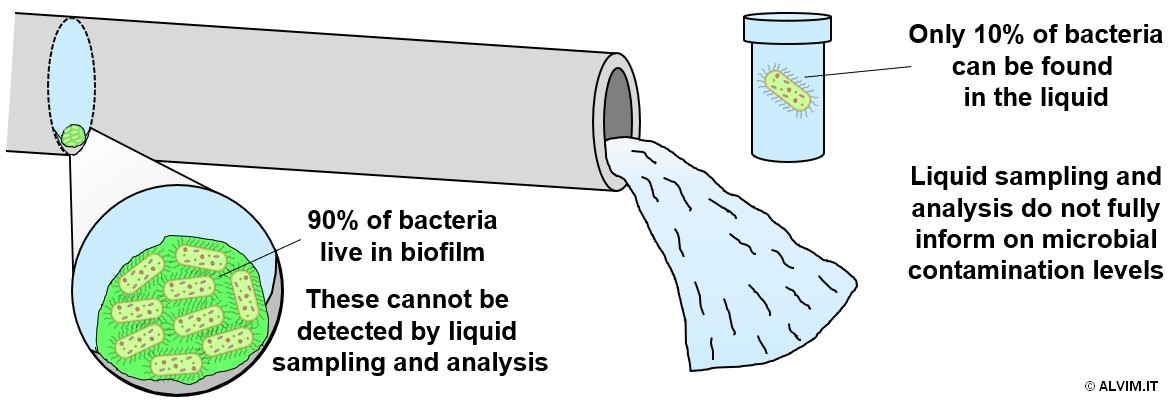
Moreover, microbial slime is the major source of microbial contamination and related problems. Therefore, it is highly important to monitor biofilm growth, in order to optimize the sanitation treatment and thus achieve an efficient and complete removal of these microorganisms. An uncontrolled biofilm growth leads to serious issues, including equipment failure, reduced filtration efficiency, increased energy consumption, microbially induced corrosion and resistance to antimicrobial treatments. Indeed, biofilm is embedded in self-produced exopolymeric substances (EPS) that shelter it from the action of biocides and other sanitizing agents. Therefore, biofilm is much more difficult to remove, compared to free-floating bacteria. It comes as a consequence that all sanitation protocols should aim expressly at biofilm removal, in order to keep microbial growth under control. The different biocide protocols available and their impact on biofilm are discussed in another white paper by ALVIM. In order to establish an efficient sanitation treatment and to get rid of biofilm, it is important to be aware of the microbial contamination of the system. For this aim, different bacteria detection methods and techniques are available, and they can be classified in different categories as shown in the following scheme.

Bacteria detection methods on the surface of pipes partially differ from the standard ones, which only allow to perform a screening in the liquid, as previously discussed. Techniques allowing to monitor biofilm offline can either lead to a quantitative or qualitative analysis; the same is true for bacteria detection in the liquid. All offline techniques require biofilm sampling, and this increases both the time and the efforts spent to perform the analysis. On the other hand, online techniques offer a great advantage, allowing to detect biofilm growth more quickly. Among these, there are many laboratory applications that are not ready for industrial use yet. Different field applications are available on the market instead, despite most of them cannot detect biofilm but only generic deposit (that includes biofilm, but also mineral scaling, etc.). This is a major limitation, because the information about generic deposit does not allow to optimize sanitation treatments. Currently, the main techniques that were demonstrated as fully able to detect biofilm in a specific and reliable way are electrochemical sensors. Among these, ALVIM Biofilm Monitoring Technologies are widely recognized as the best available option and the leading international reference for biofilm detection.
In the following paragraphs, the different categories mentioned above will be introduced, and the most commonly employed methods will be discussed in detail.
Bacteria detection in the liquid
A number of techniques are available to detect free-floating bacteria in liquid samples. In most cases, the analytical determination follows a preliminary step aimed at preparing the sample and concentrating the bacterial content. This can be achieved, for example, by means of sedimentation, ultracentrifugation or filtration and resuspension. All these operations, that shall be carried out in a dedicated laboratory, considerably increase the time required for the analysis. For bacterial enumeration, culturing techniques are widely employed, and they are often applied in the common analytical methods that allow to count colony forming units (CFU) from samples of liquid. Among these, Heterotrophic Plate Count (HPC, formerly known as Standard Plate Count, SPC, or Total Plate Count, TPC), measuring the number of viable and culturable heterotrophic bacteria, is the most common. It is important to note that these methods are often poorly representative of the real situation, since less than 1% of the total number of bacteria grows in laboratory conditions. Indeed, most bacteria are not able to grow in laboratory culturing media, and many of them fall under the category of Viable But Not Culturable (VBNC) bacteria. The same is true for bacteria detection kits, which are receiving a growing interest in the field, since their procedure is usually much easier and they allow for a faster detection even if, in some cases, their accuracy is limited.
Quantitative methods

Methods able to quantify the bacteria present in a system allow to establish a threshold above which a sanitation treatment should be applied. They do not provide further information on the kind of bacteria detected, and thus on the possible presence of hazardous pathogens (e.g. Legionella or Listeria). Quantitative methods can be further divided in two sub-categories, direct and indirect, referring to the applied technique. Direct methods work by counting or visualizing bacteria; with this respect, microscopy is probably the most widely employed. While bacteria can be visualized and counted by means of a simple compound light microscope, fluorescence microscopy offers great benefits. This technique comes with improved resolution and sensitivity. In addition, staining of the sample with fluorophores such as acridine orange or 4,6-diamidino-2-phenylindole (DAPI), which can intercalate into the DNA of bacterial cells, allows to distinguish bacteria from other substances, yielding a higher selectivity. With this respect, staining techniques also allow to distinguish between live and dead cells (e.g. green-alive and red-dead, see figure on the right). On the same principle, other assays, such as crystal-violet, can discern gram-negative from gram-positive bacteria, allowing for a general qualitative analysis. When it comes to more advanced (and also more expensive) equipment, Confocal Laser Scanning Microscopy (CLSM) can add more features. These include tridimensional view, and the possibility to simultaneously visualize different fluorescent markers, with the relative properties they highlight. In addition, an imaging software is often employed in combination with microscopy, to increase the accuracy of the analysis, and thus reduce operational efforts.
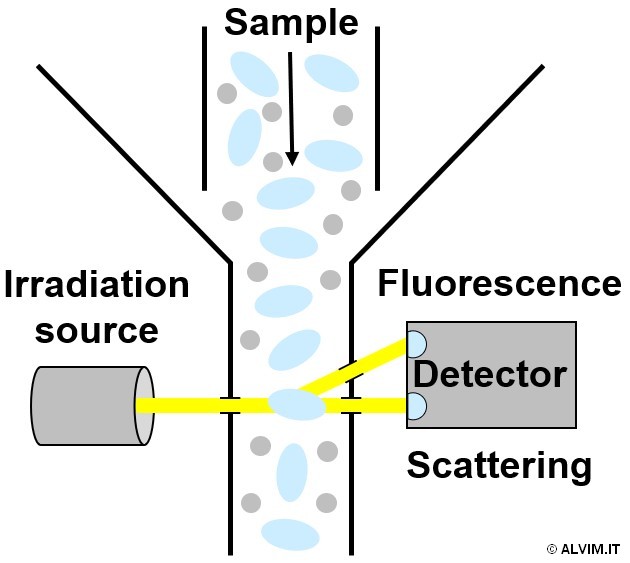
Flow cytometry is another direct method that allows to count single cells (or particles) as they flow through a narrow opening, past single or multiple irradiation beams. This technique can exploit either visible light scattering or fluorescence (see figure on the left). A similar outcome, although via a different principle, can be achieved by another direct method known as coulter counter. It allows to analyze the flow of charged particles passing in an electrolyte solution, through an opening that is part of an electrical circuit. The resulting change in voltage, determined by the movement of the particles, is related to their size. This correlation allows to distinguish and count single bacterial cells. On the other hand, indirect methods exploit a correlation of the biological phenomenon of bacterial growth with other physical parameters treated as proxy markers for bacteria. In most cases, these measurements are related to the metabolic activity of cells. For example, ATP bioluminescence (Adenosine Triphosphate - the election energy carrier nucleoside employed by living organisms) assays allow to infer about bacterial viability. With this respect, a biochemical reaction with the enzyme luciferin is exploited to convert the chemical energy deriving from ATP into visible light emission, which is spectroscopically detected. UV-Vis and fluorescence spectroscopy methods are also commonly exploited, and, when performed in conjunction with tetrazolium salts staining, they become powerful tools which find several applications in biology for monitoring metabolism in vitro.
Qualitative methods
The qualitative analysis of bacteria present in the liquid is carried out in some specific fields, including for example Food&Beverage and Oil&Gas or, less frequently, cooling systems of public and residential buildings. This is done to assess the presence of specific microorganisms, which can lead to serious issues, including the possible outbreak of water-related diseases. With this respect, the presence of Legionella, Listeria, and other pathogens in the system is commonly investigated. Depending on the required level of accuracy of the analysis, two main approaches are commonly employed. When the aim is to check for the presence of a given microorganism in the sample, test kits are exploited. They allow to perform a rapid identification which often can be done directly in the field, obtaining a positive/negative result, useful as early-warning. In addition, no laboratory equipment is needed to use most of these kits. While the indication furnished by them can be very useful, it is typically scarcely accurate. In case of positive results, further and more accurate analyses should follow. Indeed, when a more detailed investigation is needed, laboratory techniques involving the analysis of DNA are frequently employed. With this respect, the PCR (Polymerase Chain Reaction) method is the most widely employed. In this case, the genetic content of the sample is amplified, then a qualitative analysis is performed. Noteworthy, PCR based techniques can also furnish semi-quantitative results. Moreover, DNA sequencing techniques, in combination with dedicated software, can be performed for more in-depth studies, usually known as metagenomics.
Bacteria detection on surfaces
The growth and development of microbial slime on the surface of pipes can be monitored either via offline or online techniques. As anticipated above, offline biofilm detection can be performed by means of the same analytical techniques employed for free-floating bacteria. Unfortunately, most of these methods have two great disadvantages, i.e. they require a sample of biofilm and a long time to provide results. Online techniques instead eliminate both disadvantages, while offering at the same time great benefits. Indeed, monitoring biofilm online allows for a quick, or even a real-time, detection (see figure below). As a consequence, by means of online techniques it is possible to apply a timely intervention when biofilm is still young, and thus when it can be removed more efficiently.

Offline techniques
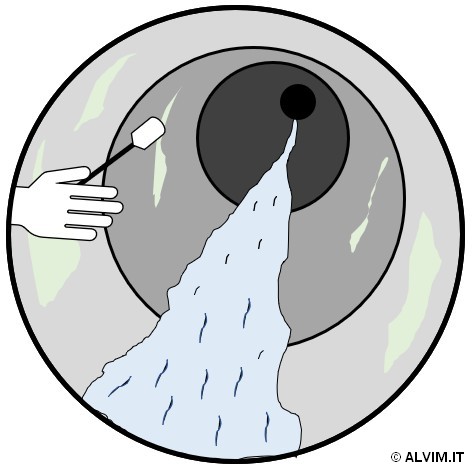
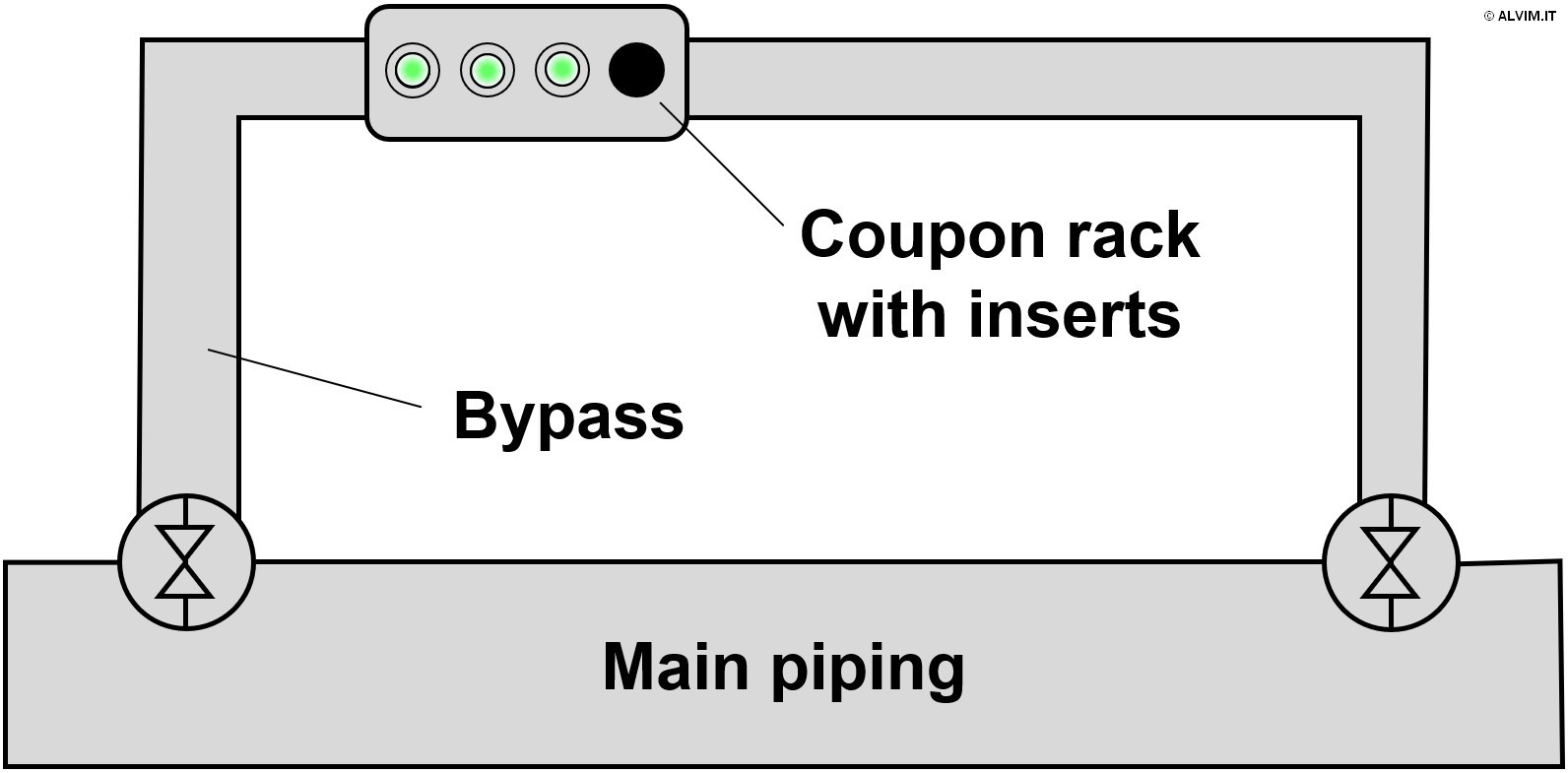
As mentioned in the previous paragraphs, sampling biofilm is required prior to offline analysis. With this respect, there are two main approaches available. The first and more direct approach consists in swabbing the internal surface of pipes, or other parts of the system, to collect a biofilm sample (see figure on the right). In order to do so, it is often necessary to interrupt operations, drain parts of the system and gain access to the internal surface of the pipes. Therefore, this approach is often difficult to put into practice, because it is expensive and time consuming. Despite the efforts required, swabbing allows to collect highly representative samples. The second, more advanced sampling technique exploits small plates of different size and material, known as coupons, which can be inserted and removed from dedicated supports. Coupons are positioned in racks, which are usually inserted in a system bypass, and biofilm is allowed to grow on their surface for a given amount of time (see figure below, on the left). Then, they are replaced, and their surface is analyzed. Noteworthy, the conditions for microbiological growth on coupons could considerably differ from those of the main piping. This can be due, for example, to the different environmental conditions in the bypass, or to the different materials present on the surface of pipelines and coupons. As a consequence, the representativeness of this kind of samples can be limited. Then, once the sample is collected, it can be submitted to either (or both) quantitative and qualitative analysis.
Quantitative methods
All of the techniques previously mentioned for the detection of bacteria in the liquid can also be applied for a quantitative, offline analysis of bacteria present on the internal surface of pipelines. While sampling is easily done in the case of liquids, more efforts are required in the case of biofilm, as discussed above. On top of the methods previously described for bacteria detection in the liquid, a number of other methods allow to detect biofilm growth in an indirect way. It is important to note that the indication provided by these methods should always be validated by means of a direct assay for biofilm detection. In addition to the ATP and UV-Vis methods described before, other common indirect methods are specifically indicated for the detection of bacteria on wet surfaces. These exploit a measure of biomass development via the determination of dry mass, total organic carbon (TOC) and total amount of proteins (see figure below).
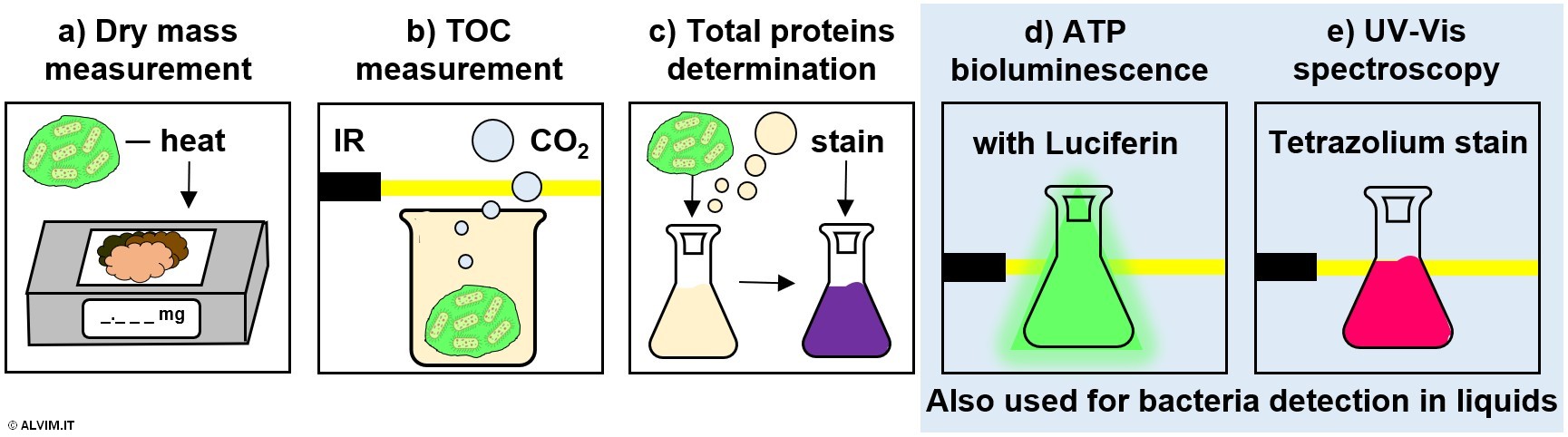
Dry mass measurement is performed by heating a biofilm sample at a constant temperature, in order to fully remove water; the remaining dry material is then weighted. Since the amount of EPS produced by bacteria differ considerably from case to case, this method might lead to misleading correlations of dry mass with biofilm growth.
The measurement of TOC is performed by disrupting biofilm via heated acidification. This process generates a volume of carbon dioxide which is proportional to the amount of bacteria and other organic matter originally present in the sample. The quantification of the produced carbon dioxide can be performed, for example, by infrared spectroscopy.
Determination of total proteins is performed by inducing the lysis of the biofilm sample in acidic or basic conditions. Following, the resulting intracellular material is stained, for example with bicinchoninic acid, Bradford or Lowry reagents. Then, a spectroscopic determination yields the final result.
Noteworthy, these techniques, which are generally destructive, do not allow to distinguish between bacteria and other microorganisms, nor between biofilm and other substances. Therefore, the use of these methods is not the most suitable choice when it comes to biofilm monitoring.
Qualitative methods
As it was the case for quantitative methods, also qualitative ones employ the same techniques that are used for bacteria detection in the liquid. The sampling step becomes in this case more delicate though, because higher accuracy and representativeness are needed.
Online techniques
Within the last decade, the online biofilm monitoring approach to microbial control started to rise in popularity and importance in the water treatment sector. While the number of applications is still limited, its adoption is expected to greatly increase in the years to come, considering the great advantages it offers. Indeed, online techniques for biofilm monitoring are the most suitable choice to achieve the optimal microbial control in a system. A variety of online methods are available, and each one of them is based on a different kind of signal that can be obtained from the biofilm under investigation. Usually relying on energy transfer principles, most of these techniques measure a passive response of the investigated surface to an input signal. Generally, these inputs are continuously transmitted to the sample, modified by the deposit (if any) and/or its environment, and then detected. Most of these methods are not specific for biofilm, so they are not able to distinguish between this bacterial layer and general deposit, or live bacteria from dead ones. This is very important to note because specific biofilm monitoring offers invaluable advantages toward the optimization of sanitation treatments.
Laboratory applications
An extensive research has been conducted, in the last few decades, on the topic of biofilm monitoring and related issues, which are recently receiving more and more attention. As a result, a number of analytical techniques were proposed, based on physical phenomena that can be related to biofilm growth. Some examples include Electrochemical Impedance Spectroscopy (EIS), vibrational and acoustic wave frequency, diffusion, piezoelectric tuning, thermic oscillation and fluorescence optics (see figure below). Following, some examples regarding the application of these techniques are reported.
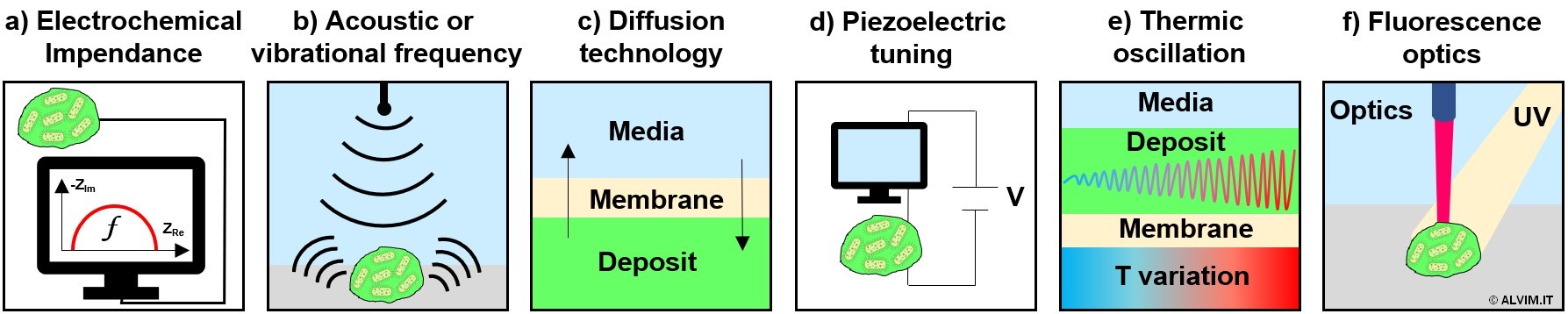
In several applications, the biomass that forms on a sensitive surface is monitored exploiting the inhibition of charge transfer between the probe and liquid media, measured by means of EIS.
The registration of vibrational or acoustic variations between liquid and solid bodies can be exploited too, allowing to infer about biofilm extent and thickness.
Another electrochemical technology able to measure biofilm thickness is based on the diffusion properties of the deposit that forms on a probe covered by a membrane.
Low frequency quartz tuning forks can be used as disposable mass sensors, to follow biofilm formation dynamics by means of piezoelectric tuning.
Another option is represented by thermal measurements, which can correlate temperature oscillations with the thickness of biofilm growing on the surface of a probe.
Last but not least, optical sensors can detect fluorescence emissions of biofilms, upon irradiation with UV light. In this case, the naturally occurring aminoacid tryptophan, which is ubiquitous in biological material, is exploited as a fluorophore. Noteworthy, this technique is reported as able to distinguish between biological matter and inorganic deposit. On the other hand, no distinction between live and dead bacteria, nor between biofilms and EPS, can be performed on this basis.
As previously discussed, all these methods are not specific for biofilm, so they are not able to distinguish between this bacterial layer and other kinds of deposit, or live bacteria from dead ones.
Field applications
A certain number of devices, aimed at biofouling or biofilm monitoring in industrial applications, can be found on the market, either as standalone products or as a part of a water treatment service. They are mainly based on visual inspection, optical properties, heat exchange capacity, ultrasonic waves and ATP measurement. Following, some examples of such devices are reported.
A basic approach is represented by fouling coupons or panels, or even transparent pipes, used to study, by naked eye or by imaging techniques, the deposit forming on the surfaces (pipes and walls) of a water system. While being able to observe macrofouling is certainly informative, this approach does not allow to detect early-stage biofilm, and thus to indicate the most appropriate timing to proceed with cleaning.
The systems based on optical sensors provide a useful indication but, in some cases, they are reported to inherently amplify the potential for biofilm growth. As a consequence, the biological phenomenon detected by the optical system might be altered, and thus it might lead to an overestimate. It is also worth mentioning that online optical technologies lack the ability to distinguish between biofilm and general deposit.
Other commercial devices allow to infer about biofilm growth by measuring the heat exchange capacity of deposit formed on the surface of a probe. As discussed above, also in this case general deposit is monitored, not biofilm.
Ultrasonic probes are commercially available too, for the detection of deposit build-up. Although these devices are able to distinguish between hard and soft fouling, they cannot discern biofilm from other soft deposits. In addition, this monitoring technique suffers from the influence of many interferences, including small fluctuations in water temperature.
Last but not least, some ATP based applications are also available. These offer the advantage of a specific detection of biological over non-biological material. On the other hand, they do not operate strictly online, since sampling, however automatic and fast, is always necessary. As a consequence, these techniques are rather complex, and they are characterized by both high OPEX and CAPEX.
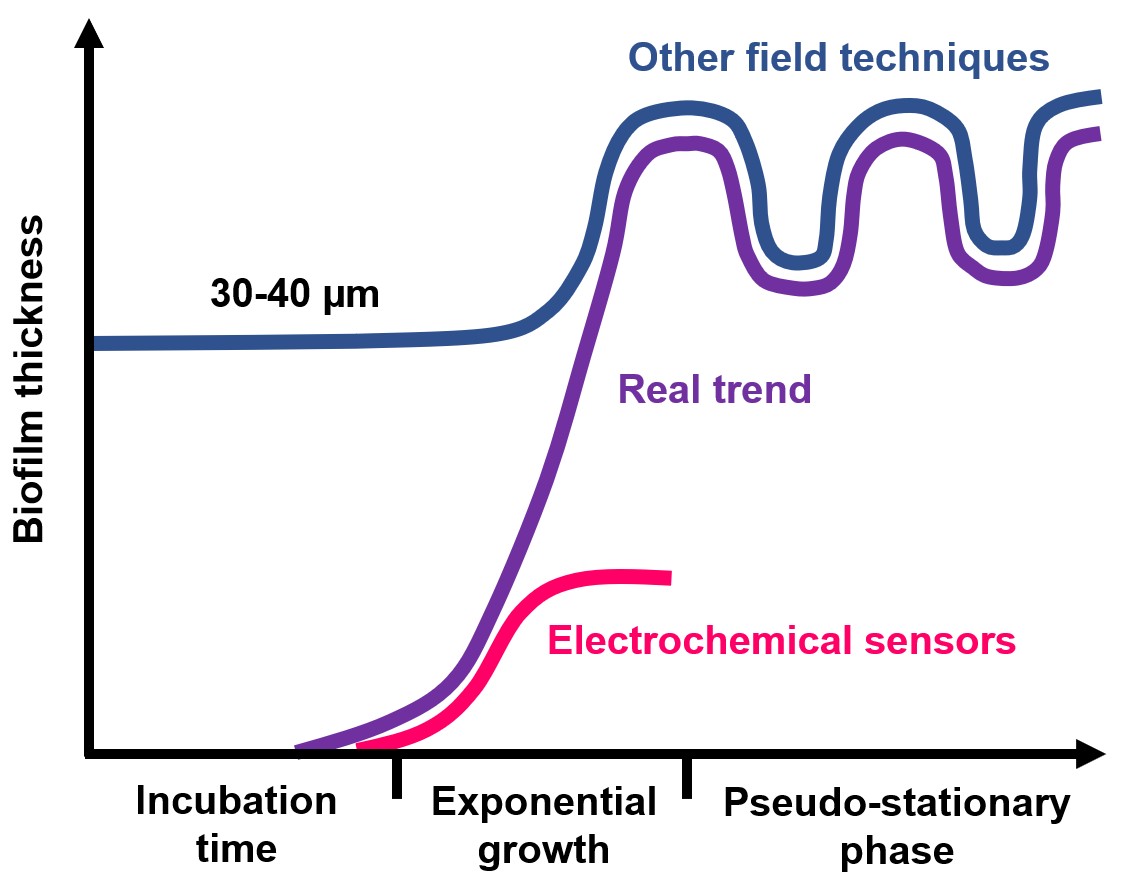
Currently, the technologies scientifically proven as able to specifically detect biofilm, most widely applied in many industrial applications, are based on the measurement of the electrochemical activity of bacteria. In addition, when compared to other field techniques, electrochemical sensors are far more sensitive toward biofilm. Indeed, they can detect the formation of the very first layer of bacteria (with a thickness below 5 µm) while the limit of detection of other field techniques is well above 30 µm (see figure on the right).
With this respect, the ALVIM System is by far the most widely known, validated and used biofilm monitoring device, worldwide. The ALVIM system is currently employed by the largest Companies operating in the Food&Beverage, Pulp&Paper, Oil&Gas and Energy production.
Before ALVIM, other electrochemical sensors, based on a completely different techniques, have been commercialized by other Companies. While based on an electrochemical principle too, in that case the basic phenomenon was never fully studied nor validated. The occasional lack of reliability and stability under small fluctuations of temperature and flow, strongly limited the application of those technologies. Additionally, in some cases those devices generate, on their surface, an environment that promotes biofilm growth. Therefore, the indication of biofilm growth given by the device cannot be considered as fully representative of what takes place in the real process.
On the contrary, ALVIM Sensors detect the real biofilm growth, without affecting it. The working principle behind the ALVIM System, known as 'cathodic depolarization' or 'ennoblement', has been extensively studied and validated, over more than 40 years of both public and independent research. ALVIM Sensors specifically detect the bioelectrochemical activity of bacterial biofilm, making it possible to monitor the phenomenon since the very first phase - with a sensitivity often higher than that of most laboratory techniques. As a result, an 'early-warning' signal is provided - extremely useful, since the very first phase of biofilm is the easiest to remove.
To sum-up, the ALVIM System offers, at the same time, all of the most important features to solve biofilm related issues:
- online and real-time monitoring,
- specific biofilm detection,
- high sensitivity (early warning signal).
|
Do you have biofilm-related issues?
|





